Abstract
The mountain birch [Betula pubescens var. pumila (L.)] forest in the Subarctic is periodically exposed to insect outbreaks, which are expected to intensify due to climate change. To mitigate abiotic and biotic stresses, plants have evolved chemical defenses, including volatile organic compounds (VOCs) and non-volatile specialized compounds (NVSCs). Constitutive and induced production of these compounds, however, are poorly studied in Subarctic populations of mountain birch. Here, we assessed the joint effects of insect herbivory, elevation and season on foliar VOC emissions and NVSC contents of mountain birch. The VOCs were sampled in situ by an enclosure technique and analyzed by gas chromatography–mass spectrometry. NVSCs were analyzed by liquid chromatography–mass spectrometry using an untargeted approach. At low elevation, experimental herbivory by winter moth larvae (Operophtera brumata) increased emissions of monoterpenes and homoterpenes over the 3-week feeding period, and sesquiterpenes and green leaf volatiles at the end of the feeding period. At high elevation, however, herbivory augmented only homoterpene emissions. The more pronounced herbivory effects at low elevation were likely due to higher herbivory intensity. Of the individual compounds, linalool, ocimene, 4,8-dimethylnona-1,3,7-triene, 2-methyl butanenitrile and benzyl nitrile were among the most responsive compounds in herbivory treatments. Herbivory also altered foliar NVSC profiles at both low and high elevations, with the most responsive compounds likely belonging to fatty acyl glycosides and terpene glycosides. Additionally, VOC emissions from non-infested branches were higher at high than low elevation, particularly during the early season, which was mainly driven by phenological differences. The VOC emissions varied substantially over the season, largely reflecting the seasonal variations in temperature and light levels. Our results suggest that if insect herbivory pressure continues to rise in the mountain birch forest with ongoing climate change, it will significantly increase VOC emissions with important consequences for local trophic interactions and climate.
Keywords: biotic stress, geometrid moth, global change, plant–insect interactions, secondary metabolites, volatile organic compounds
Introduction
Plants are constantly under attack from herbivores and other biotic stresses (Trowbridge and Stoy 2013, Kutchan et al. 2015). To combat herbivory attack, plants have evolved a diverse arsenal of chemical defenses, including production of volatile organic compounds (VOCs) and non-volatile specialized compounds (NVSCs; Trowbridge and Stoy 2013, Kutchan et al. 2015). In response to herbivore attack, plants often increase biosynthesis and production of VOCs and NVSCs, and alter chemical compositions of these compounds (Kesselmeier and Staudt 1999, Howe and Jander 2008, Dicke et al. 2009, Tholl et al. 2011, Hartikainen et al. 2012, Hammond-Kosack and Jones 2015, Ameye et al. 2018).
Herbivore-induced chemical defenses are divided into direct and indirect defenses (Haukioja 2005, Turlings and Erb 2018). The direct defenses can be mediated by VOCs or toxic NVSCs, inhibiting the colonization, growth, reproduction, development and survival of herbivores (Trowbridge and Stoy 2013, Wang and Wu 2013, Hammond-Kosack and Jones 2015). As indirect defenses, VOCs can recruit natural enemies of herbivores, such as predators and parasitoids (Turlings and Erb 2018), and NVSCs might reduce the immunocompetence of insect herbivores, and thus impede herbivores’ defenses against predators and parasitoids (Haukioja 2005). Volatile organic compounds and NVSCs can also protect plants from abiotic stresses (Loreto and Schnitzler 2010, Kutchan et al. 2015). For example, many VOCs such as isoprene and monoterpenes (MTs), and NVSCs such as phenolics, have been shown to operate as antioxidants and protect plants against oxidative stress caused by high temperatures, elevated tropospheric ozone levels and excess light exposure (Loreto and Schnitzler 2010, Kutchan et al. 2015). In addition to their ecologically relevant roles, VOCs are highly reactive in the atmosphere and can drive climate feedbacks via complex atmospheric chemistry (Peñuelas and Staudt 2010, Loreto and Fares 2013, Faiola and Taipale 2020).
Research on constitutive and herbivore-induced responses of specialized compounds often shows substantial seasonal variation in their magnitude (Peñuelas and Llusià 1999, Hakola et al. 2001). For example, the VOC emission patterns were observed to change substantially over the growing season in many plant species, such as Quercus ilex (Peñuelas and Llusià 1999), Datura wrightii (Hare 2010), Betula spp. (Hakola et al. 2001) and Pinus edulis (Trowbridge et al. 2014). Although VOC emission rates seem to increase progressively throughout the active growing season and decline during leaf senescence, large variabilities in the seasonal patterns occur between compounds and plant species. Such high seasonal variation has been thought to be caused by variation in environmental factors (e.g., solar radiation, temperature and humidity) that vary over the growing season and can influence production of specialized compounds, and/or by ontogenetic changes in leaf physiology (Hare 2010, Niinemets et al. 2010, Li et al. 2019).
Rising temperatures, due to climate change, are generally expected to increase VOC emissions, because their biosynthesis and release are temperature dependent (Holopainen et al. 2018). However, negative and neutral effects of temperature on VOC emissions have also been reported (Peñuelas and Staudt 2010, Holopainen et al. 2018). Moreover, the magnitude of the temperature influences on VOC emissions varies among compounds, depending on the physicochemical properties of the compounds, the presence of specialized storage pools and the co-occurrence of other environmental factors (Peñuelas and Staudt 2010, Li et al. 2019). For instances, previous studies have shown that some highly volatile compounds like isoprene and non-oxygenated MTs are more responsive to temperature than the less volatile compounds like oxygenated MTs and fatty acid-derived green leaf volatiles (GLVs), especially when they are released from plant species lacking storage structures (Peñuelas and Llusià 1999, Peñuelas and Staudt 2010). Likewise, the foliar contents of different classes of NVSCs may respond differently to warming (Holopainen et al. 2018). Furthermore, increasing temperatures may enhance defoliation pressure exerted by insect herbivore infestation (Bale et al. 2002). Higher latitude regions are expected to experience larger temperature increases compared with lower latitudes (IPCC 2014) and as such, higher latitude regions are likely to experience more pronounced increases in defoliation pressure (Bale et al. 2002) and VOC emissions (Rinnan et al. 2014). However, the simultaneous effects of rising temperatures and concomitant increased insect herbivore infestation pressure on plant chemical defense mechanisms and the susceptibility of plants to herbivores are poorly understood (Descombes et al. 2020), especially in the Arctic regions.
Here, we aimed to assess how the interactive effects of climate change and insect herbivory influence plant specialized compounds in the mountain birch [Betula pubescens var. pumila (L.)] forest that dominates the tree line zone in Subarctic Fennoscandia (Ammunet et al. 2015). We investigated in situ responses of VOCs and NVSCs to insect herbivory at two elevations during a growing season. Natural climatic conditions change with elevation and environmental variables, such as declining temperature with altitude, and allow us to investigate the effects of climate change on plant chemical defense mechanisms (Körner 2007, Moreira et al. 2018). We hypothesized that the VOC emission rates and foliar NVSC contents would (i) increase in response to herbivory in accordance with previous studies (Hartikainen et al. 2012, Ossipov et al. 2014, Li et al. 2019, Rieksta et al. 2020); (ii) differ between elevations in relation to temperature differences (Close and McArthur 2002, Körner 2007, Loreto and Fares 2013); and (iii) vary throughout the growing season as a result of seasonal variations in plant growth activity, phenology and the microclimate, such as air temperature and photosynthetically active radiation (Haukioja 2003, Loreto and Fares 2013).
Materials and methods
Study area and experimental design
The mountain birch forest covers the ecotone between the coniferous forest zone and the treeless area beyond the Arctic, alpine and maritime tree lines (Wielgolaski 2005), ranging through climatic gradients from the mild oceanic regions of northwestern Norway, to cold and dry areas in northern Sweden and Finland (Ammunet et al. 2015). These mountain birch forests are exposed to defoliation pressure exerted by geometrid moth (Lepidoptera: Geometridae) species: the winter moth (Operophtera brumata), autumnal moth (Epirrita autumnata) and umber moth (Agriopsis aurantiaria). The three geometrid moth species are considered the most important natural disturbance in mountain birch forests influencing ecosystem dynamics and processes, such as carbon and nitrogen cycling (Tenow and Bylund 2000, Ammunet et al. 2015).
Our study was conducted in a mountain birch forest at Kvaløya in Tromsø, Norway, in the growing season of 2018. Tromsø has a Subarctic climate with a mean annual temperature of +2.9 °C and a mean annual precipitation of 1011 mm (1925–2013), with snow melt as late as the end of May (Opala et al. 2016). Mountain birch is the dominant vegetation in the tree layer (Opala et al. 2016) and winter moth, autumnal moth and umber moth all naturally occur in this area. The experiment consisted of two elevational transects (69°41′N, 18°47′E and 69°41′N, 18°49′E) on a southern slope, ~2.5-km apart. Each transect comprised two sites, one at the low elevation and the other at the high elevation, i.e., 50 and 240 m above sea level, respectively. The two sites at the low elevation, were established at the lower extent of the mountain birch forest, and the high elevation sites were established at the upper tree line.
In the beginning of the experiment in late May, we selected eight pairs of trees at each site, and on each tree, labeled a sun-lit branch with no visible sign of herbivory or disease ~1.5-m above ground. We then enclosed the distal 25–35 cm of the labeled branches in transparent mesh bags to protect them from natural insect herbivores. The branches were later assigned for experimental herbivory manipulation and specialized compound measurements. Because the whole birch stand at the low elevation sites suffered from an aphid infestation, aphids were gently removed with a soft paintbrush before the branches were enclosed in mesh bags. In total, we selected 64 branches (i.e., 2 transects × 2 elevations at each transect × 8 tree pairs at each elevation). The target parts of the branches contained on average 71 ± 3 leaves [mean ± standard error (SE), n = 64] ranging from 30 to 126 leaves. To take into account the potential confounding effects of the large variation in branch size on herbivore-induced specialized compound production, we paired the branches with similar numbers of leaves and applied herbivory treatment randomly to the two branches within each pair [control branches: 69 ± 4 leaves ranging from 30 to 123 (n = 32); infested branches: 73 ± 4 leaves ranging from 38 to 126 (n = 32)].
For the herbivory treatment, we used winter moth larvae that were collected in their first to second instar in northeast Finnmark (70°9′N, 28°51′E) and reared on detached birch branches in the laboratory. The winter moth populations in the mountain birch forest exhibit dramatic interannual variation. The number of larvae on a branch of ~60- to 80-cm long can vary from <1 during non-outbreak years to >100 during outbreak years (Pepi et al. 2017). In the beginning of the experiment, the low and high elevation sites differed in phenology; all leaves were unfolded at low elevation, whereas leaves had just started to unfold at high elevation. Due to the differences in leaf phenology between the elevations and in order to assess the herbivory effects over the entire feeding period, which usually lasts from late May/early June to late June/early July in the mountain birch forest, we applied three and five winter moth larvae to the high and low elevation sites, respectively, which fall well within the observed natural variation. The larvae were in their third to fourth instar and not starved when applied to the branches. The herbivory treatment lasted for ~3 weeks from mid-June to early July (see Table S1 available as Supplementary data at Tree Physiology Online for the exact start and end dates). During this feeding period, VOCs were collected three times (see below for detailed procedures). Before VOC collection, we gently removed mesh bags as well as larvae and larval feces from the leaves using a soft paintbrush. After VOC collection, we placed larvae back, along with the mesh bag, and replaced the dead or missing larvae with new ones so that the infested branches at high and low elevations still received three and five larvae, respectively. Different numbers of larvae were used corresponding to the differences in leaf and tree sizes at the two altitudes and targeting similar leaf area losses as a result of herbivory. Similar percentage leaf area losses were observed across elevations at the end of herbivory treatment period suggesting minimal confounding effects from the different larval numbers.
Volatile organic compound sampling
Volatile organic compounds were sampled using the push–pull enclosure technique (Ortega and Helmig 2008). Enclosures were pre-cleaned (120 °C for 1 h) polyethylene terephthalate (PET) oven bags (35 × 43 cm), which were ventilated at a flow rate of 1000 ml min−1 for a minimum of 10 min prior to VOC sampling. The inflow air was purified by a charcoal filter (F03-C2-100, Filter: MXP-95-054, Wilkerson Corp., Richland, Michigan, USA) and potassium iodide ozone scrubber (Ortega and Helmig 2008). During the 15-min sampling period, with an inflow rate of 300 ml min−1 and outflow rate of 200 ml min−1 (3-l sample volume), VOCs were trapped by stainless-steel adsorbent cartridges containing 150-mg Tenax TA and 200-mg Carbograph 1TD (Markes International Ltd, Llantrisant, UK). After sampling, cartridges were sealed with Teflon-coated brass caps and stored at 5 °C until analysis. Blank measurements from empty PET bags were performed to obtain information on impurities originating from the sampling or analysis system. During VOC sampling, the air temperature and humidity at 1.5-m above ground and inside the PET bags was recorded every minute by shaded iButtons (Hygrochron, DS 1923-F5, Maxim Integrated Products Inc., San Jose, California, USA). Photosynthetic photon flux density (PPFD) was recorded every 10 s by S-LIA-M003 sensors (Onset Computer Corporation, Bourne, Massachusetts, USA) coupled with a Hobo Micro Station (Onset Computer Corporation, Bourne, Massachusetts, USA).
Five VOC sampling campaigns were conducted at each site in each elevation transect (see Table S1 available as Supplementary data at Tree Physiology Online). The first campaign was carried out 2–6 June, herein referred to as the ‘early’ measurement (see Table S1 available as Supplementary data at Tree Physiology Online). Herbivory treatments started 8–13 June and ended 1–5 July, comprising the defoliation period (see Table S1 available as Supplementary data at Tree Physiology Online). Three VOC campaigns were carried out during the defoliation period, herein referred to as the ‘mid-1’, ‘mid-2’ and ‘mid-3’ measurements (see Table S1 available as Supplementary data at Tree Physiology Online). The mid-1 measurement was carried out 13–17 June, the mid-2 measurement 25–30 June and the mid-3 measurement 1–5 July (see Table S1 available as Supplementary data at Tree Physiology Online). The final campaign was carried out 10–11 August, which was 5 weeks after the termination of the herbivory treatment, herein referred to as the ‘late’ measurement (see Table S1 available as Supplementary data at Tree Physiology Online). The VOC sampling took place over multiple days because it was not possible to complete all measurements in a single day and work sometimes was delayed by unfavorable weather conditions. At each transect, VOC sampling was carried out almost concurrently at the two elevations, except on 1 July when the measurements were first completed at the high and then the low elevation site.
The approximate leaf area (m2) per branch after the first four campaigns, as well as the leaf damage per branch, i.e., leaf area consumed by larvae, after each campaign within the defoliation period, was visually estimated as in Li et al. (2019). Branches were cut after the last campaign and leaf area was calculated as in Li et al. (2019). The VOC emission rates were calculated based on the estimated and calculated leaf areas per branch after each campaign. Leaf dry mass per unit area was 50.3 ± 0.8 g m−2 (n = 18).
Volatile organic compound analysis
The VOCs were thermally desorbed (TD100-xr, Markes International Ltd, Llantrisant, UK) from the adsorbent cartridges at 250 °C for 10 min and analyzed on a gas chromatograph–mass spectrometer (7890A GC, 5975C VL MSD, Agilent Technologies, Santa Clara, California, USA). The VOCs were first cryofocused at −10 °C and injected into a HP-5MS capillary column (50 m × 0.2 mm, film thickness 0.33 μm), with helium as carrier gas at a flow rate of 1.2 ml min−1, and separated using the following temperature program: 40 °C, held 1 min; raised at 5 °C min−1 to 210 °C; raised at 20 °C min−1 to 250°, held 8 min.
Chromatograms were analyzed using PARADISe v. 3.8 (Johnsen et al. 2017). Compounds were identified using pure standards, when available, or tentatively identified using the Mass Spectral Library (NIST 2014). Compounds were categorized into the following classes: MTs, homoterpenes (HTs), sesquiterpenes (SQTs), GLVs, isoprene and other VOCs (OVOCs, i.e., compounds that were not included in any of the above-mentioned groups). When pure standards were not available, quantification was achieved using the following pure standards: for MTs and HTs: α-pinene; SQTs: caryophyllene; GLVs: hexenal; and OVOCs: toluene. Compounds were included in the dataset if they were present in a minimum of 50% of the samples in at least one treatment group and had a match factor above 800 against the Mass Spectral Library (NIST 2014). The VOC concentrations in blanks were subtracted from those in the samples. The leaf area-based emission rates were calculated in accordance with Ortega and Helmig (2008).
Non-volatile specialized compound sampling
From each branch, five leaves at the low and three leaves at the high elevation sites were harvested before and after the defoliation period, i.e., during the period 8–13 June and 2–6 July, respectively (see Table S1 available as Supplementary data at Tree Physiology Online). The leaves harvested before the defoliation period were picked from neighboring branches on the same tree, and the leaves harvested after the defoliation period were only picked if marked by larvae chewing. Leaf samples were kept on ice during local transportation to the laboratory and stored in a −80 °C freezer, but all samples were unintentionally defrosted for a maximum of 24 h during transportation to Copenhagen.
The effect of the defrosting event on foliar NVSC contents was assessed by comparing NVSC contents in different mountain birch leaves with and without a similar defrost treatment. The defrost treatment significantly affected the foliar contents of a considerable number of the tested NVSCs: 30% of NVSCs decreased and 7% increased (P < 0.05, T-test). All the samples collected during our experiment were stored and transported together, under the same conditions and as such, we decided to investigate changes in the proportional foliar NVSC contents, rather than reporting the absolute quantities.
Non-volatile specialized compound analysis
Frozen leaves were ground in liquid nitrogen with a mortar and pestle and homogenized tissue (~0.03 g) was boiled (5 min at 90 °C) in 500 μl of extraction buffer (85% methanol, 0.1% formic acid and 250 μM Amygdalin as internal standard), subsequently cooled on ice (10–20 min), and centrifuged (4 °C, 15,000 r.p.m., 5 min; dos Santos et al. 2019). The supernatant was diluted five times and filtered through a membrane filter (0.45 μm, Merck Millipore, Burlington, Massachusetts, USA) before being stored at 4 °C until further analysis (dos Santos et al. 2019). Sample dry weight was obtained from the dried pellets (60 °C for 24 h).
Chromatographic separation of the filtered samples was performed on a Dionex Ultimate 3000RS ultra-high performance liquid chromatograph (UHPLC; Thermo Fisher Scientific, Waltham, Massachusetts, USA) system with a diode array detector, cooling autosampler at 10 °C, and column oven at 40 °C. Five microliters of every sample were separated, using a Phenomenex Kinetex® column (100 Å, 1.7 μm C18, 150 × 2.1 mm, Phenomenex Inc., Torrance, California, USA), by gradient elution, at a constant flow rate of 0.3 ml min−1. The gradient elution profile was as follows: 98% solvent A (0.05% formic acid in water) and 2% solvent B (0.05% formic acid in acetonitrile) for 3 min, linearly increasing to 40% solvent B over 12 min, then 60% solvent B over 5 min, 100% solvent B over 10 min and held for 10 min before solvent B was decreased to 2% over 5 min and the column was re-equilibrated for a further 5 min. The UHPLC system was coupled with a compact™ mass spectrometer (Bruker Daltonics, Billerica, Massachusetts, USA) with an electrospray ionization source. The mass spectrometer detected compounds in negative mode in the range m/z 50–1400 as in dos Santos et al. (2019). All samples were run in full scan mode followed by the auto tandem mass spectrometry (MS/MS) mode having a bbCID collision energy of 20 eV.
Data acquisition and calibration was performed using the Bruker Compass Data Analysis v. 4.3 (Bruker Daltonik Gmbh 2014, Billerica, Massachusetts, USA) software. Raw chromatogram data were subsequently exported as .mzML format and further processed using MZmine2 (v.2.5.3; Pluskal et al. 2010). A principal component analysis was conducted that revealed narrow clustering of pooled samples and blanks, indicating overall reliable spectral data acquisition throughout the analysis (see Figure S1 available as Supplementary data at Tree Physiology Online). The chemical annotation pipeline was conducted as described in Gericke et al. (2020). An in-house MS/MS spectral database was generated including 18 commercially sourced reference compounds (see Table S2 available as Supplementary data at Tree Physiology Online). The three levels (1–3) of identification that describe confidence in dereplication events in this study are in line with the refined levels by the Metabolomics Standards Initiative of the Metabolomics Society (Schymanski et al. 2014). A detailed description of the MZmine2 processing as well as molecular networking parameters used in this study can be found in Table S3 and Figure S2, available as Supplementary data at Tree Physiology Online. Individual metabolites were selected for further identification via SIRIUS (v4.5.1) under consideration of M−H− adducts while using default settings (Dührkop et al. 2015, 2019, 2020, Böcker and Dührkop 2016). A total of 449 NVSCs were detected in our untargeted UHPLC–MS/MS analysis. This resulted in the annotation of 63 metabolites based on levels 1 and 2 identification. Of the 449 NVSCs, 235 were included in the dataset after removing the compounds present in <6% of the samples.
Statistical analyses
All statistical analyses were executed using R statistical framework version 3.6.1 (R Development Core Team 2019). We assessed the effects of herbivory, elevation, measurement time and their interactions on VOC emissions, foliar NVSC contents and leaf damage using mixed-effect models (LMM) fitted with maximum likelihood using the ‘lmer’ function from the lme4 package (Bates et al. 2015). Treatment, elevation and measurement time were predictor variables and transect, site, branch pair and branch were random factors. Analysis of variance (ANOVA) was used to test for overall effects of the predictor variables on all of the response variables. T-tests were used on all of the response variables to test for differences between the control and herbivory treatments in each measurement time at each elevation, as well as differences between elevations in each measurement time, using the `difflsmeans' function from the lmerTest package (Kuznetsova et al. 2017). T-tests were also used to test for significant differences between measurement times for the foliar NVSC contents. Tukey’s post-hoc tests were used to test for significant differences in VOC emissions between the measurement times using the `emmeans' function from the emmeans package (Russell 2019). Similar methods were used to test for differences in temperature and PPFD between treatments, elevations and measurement times. To fulfill the requirements of normal distribution and homogeneity of variance, a log(x + 1, raised to the power of the exponent of the lowest value not equal to zero) was applied on all the response variables, except for the enclosure temperature.
Due to the temperature and light dependencies of VOC emissions (Loreto and Fares 2013), we assessed whether the temporal variations in the measured VOC emissions were connected to the temporal variation in the enclosure temperature and PPFD by Pearson’s correlation test using the `cor.test' function.
The machine learning algorithm Random Forest (RF) was used to assess whether the blends of VOC emission rates and the proportional foliar NVSC contents could be separated by the treatments, elevations and measurement times. The precision of RF to separate groups is assessed with the RF prediction error, i.e., out-of-bag error (OOB; Breiman 2001, Ranganathan and Borges 2010). Furthermore, RF was used to obtain the variable importance (E) table, which shows individual VOCs and NVSCs that are most important for separation between treatments, elevations and measurement times (Breiman 2001, Ranganathan and Borges 2010). We used the `randomForest' function from the randomForest package (Liaw and Wiener 2002) with an ntree = 100.000, and an mtry = 12 for VOCs and mtry = 16 for NVSCs (Breiman 2001). The RF analyses were performed for each measurement time at each elevation for both VOCs and NVSCs.
Results
Leaf damage from herbivory treatment
The herbivory treatment progressively increased leaf damage over time, leading to ~9% leaf area loss by the mid-3 measurement (Figure 1). There was a significant interaction effect between elevation and measurement time on leaf damage. Percentage leaf area losses were significantly higher at low than high elevation during the early herbivory period (i.e., 6% versus 2%), but similar between the two elevations both in the middle and the end of the herbivory period (Figure 1).
Figure 1.
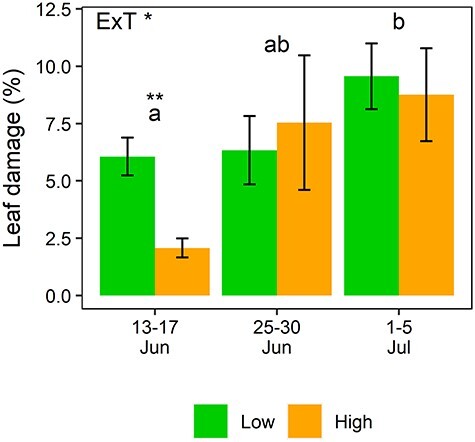
Leaf damage on herbivory treated branches at low and high elevations. The label (ExT *) denotes a significant interaction between elevation and measurement time (P < 0.05, ANOVA). The asterisks above the bars indicates a significant difference between the elevations (P < 0.01, T-test). Letters above bars denotes significant differences between measurement times (P < 0.05, Tukey’s post-hoc test). Bars represent mean ± SE (n = 16).
Variation in temperature and PPFD
Air temperature differences between the two elevations were in general low (see Figure S3 available as Supplementary data at Tree Physiology Online). The temperature difference was the largest in the early growing season when the monthly air temperatures were 9.1 ± 0.5 °C (mean ± SE) for May and 7.6 ± 0.5 °C for June at low elevation, and 1.1 °C cooler at the high elevation. In July, the monthly air temperatures were similar at both elevations, on average 16.3 ± 0.7 °C. In August and September, the temperature differences between the elevations for the monthly means were 0.7 °C.
The enclosure temperature in VOC measurements was 1.5 °C higher at the low compared with the high elevation during the early measurement (P < 0.01 for elevation × time interaction; see Figure S4A available as Supplementary data at Tree Physiology Online). There was no effect of elevation on PPFD (see Figure S4B available as Supplementary data at Tree Physiology Online). Both enclosure temperature and PPFD varied substantially across the season, being higher at mid-1, mid-3 and late measurement times, and lower at early and mid-2 measurement times (P < 0.05, Tukey’s post-hoc test; Figure 2A; see Figure S4A and B available as Supplementary data at Tree Physiology Online).
Figure 2.
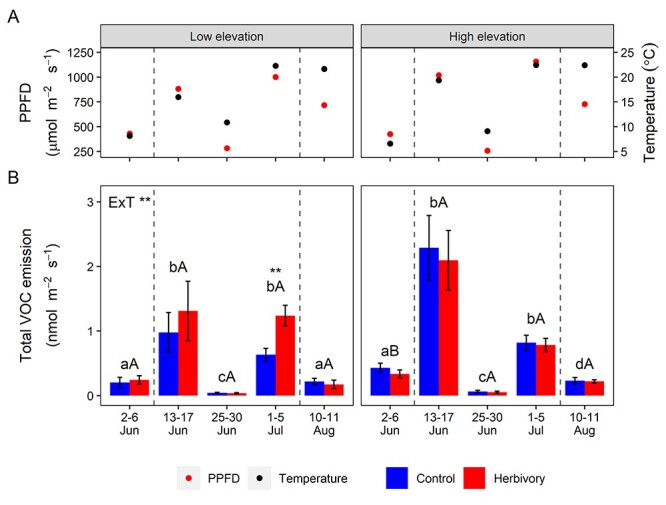
Total VOC emissions from control and herbivore-damaged branches at low and high elevations. The mean PPFD and enclosure temperature (A) and total VOC emission from control and herbivory treated branches (B) measured five times during the growing season at low and high elevations. The label (ExT **) denotes a significant interaction between elevation and measurement time (P < 0.01, ANOVA). The asterisks above the bars indicate a significant difference between the control and herbivory treatment within each measurement time and elevation (P < 0.01, T-test). Bars labeled with different lowercase letters indicate significant differences between measurement times at the same elevation (P < 0.05, Tukey’s post-hoc test). Bars labeled with different uppercase letters denotes significant differences between elevations within each sampling time (P < 0.05, T-test). Bars represent mean ± SE (n = 16; n = 15 for the control at high elevation 2–6 June). Dashed lines indicate the defoliation period.
Herbivory effects on VOC emissions
A total of 130 VOCs were detected, consisting of 22 MTs, 49 SQTs, 2 HTs, 11 GLVs and isoprene, and 45 OVOCs containing alcohols, aldehydes, alkanes, alkenes, benzenoids, furans, ketones and nitrogen-containing compounds (see Table S4 available as Supplementary data at Tree Physiology Online). Sesquiterpenes, GLVs, OVOCs, MTs, isoprene and HTs accounted for 62, 17, 11, 8, 0.8 and 0.5% of total VOC emissions, respectively.
There were no overall significant herbivory effects on total VOCs or most VOC classes (Figures 2B; and 3; see Figure S5 available as Supplementary data at Tree Physiology Online). However, there were significant interaction effects between herbivory treatments, elevation and sampling time on the MT and HT emissions (Figure 3A and B). Furthermore, clear herbivory effects were seen on emissions of MTs and HTs during the defoliation periods, especially at low elevation (Figure 3A and B). At each of the three sampling times during the defoliation period, herbivory at low elevation significantly increased MT and HT emissions, which, when averaged across sampling times, were 300 and 590% higher in defoliated branches than control branches, respectively. At low elevation, the herbivory treatment also increased the proportional contributions of the MTs and HTs to the total VOC emission by an average of 230 and 400%, respectively, during the defoliation period (see Figure S6 available as Supplementary data at Tree Physiology Online). Moreover, at low elevation, herbivory substantially increased emissions of total VOCs, GLVs, SQTs and OVOCs, by 200, 300, 200 and 60%, respectively, at the end of the defoliation period (Figures 2B and 3C,D; see Figure S5A available as Supplementary data at Tree Physiology Online). By contrast, herbivory at high elevation sites significantly influenced only HT emissions, with 270% higher emissions observed in defoliated branches (Figure 3B). There was no effect of herbivory treatment on isoprene emissions (see Figure S5B available as Supplementary data at Tree Physiology Online).
Figure 3.
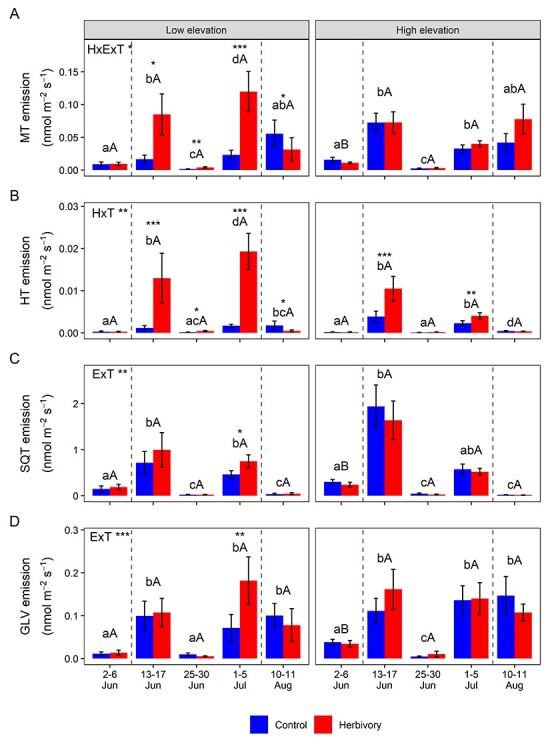
MT, HT, SQT and GLV emissions from control and herbivore-damaged branches at low and high elevations. MT (A), HT (B), SQT (C) and GLV (D) emissions from control and herbivory treated branches at five measurement times during the growing season at low and high elevations. Significant effects and interactions for herbivory (H), elevation (E) and measurement time (T) are shown (*P < 0.05; **P < 0.01; ***P < 0.001; ANOVA). The asterisks above the bars indicate a significant difference between the control and herbivory treatment within each sampling time and elevation (*P < 0.05; **P < 0.01; ***P < 0.001; T-test). Bars labeled with different lowercase letters indicate significant differences between measurement times at the same elevation (P < 0.05, Tukey’s post-hoc test). Bars labeled with different uppercase letters denotes significant differences between elevations within each sampling time (P < 0.05, T-test). Bars represent mean ± SE (n = 16; n = 15 for the control at high elevation 2–6 June). Dashed lines indicate the defoliation period.
According to the RF analysis, differences in the VOC blends between herbivory and control treatments were most evidently separated at the end of the defoliation period (mid-3) at the low elevation (OOB: 18.75%; Figure 4A). Ten compounds were ranked as most important for discriminating between control and herbivory treatments, including five MTs, two HTs, one SQT and four OVOCs, all of which were released in significantly higher amounts from defoliated branches (Table 1).
Figure 4.
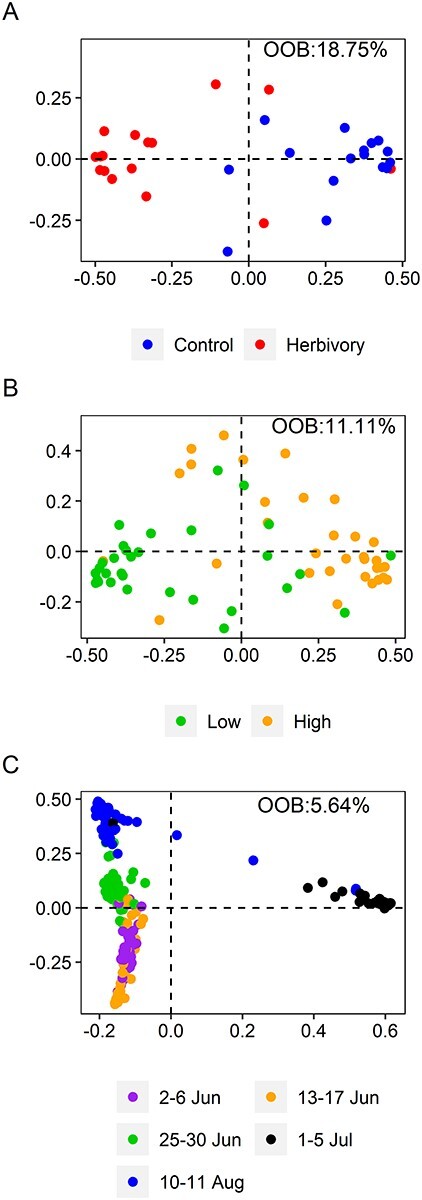
Metric multi-dimensional scaling (MDS) plots of the results of the RF analyses on VOCs. MDS plots of the proximity matrices showing the separation of the samples according to the VOC blends, between the control and herbivory treatment in the end of the defoliation period, i.e., 1–5 July, at the low elevation (A), between low and high elevations across herbivory treatments in the early growing season, i.e., 2–6 Jun (B), and among measurement times across both herbivory treatments and elevations (C).
Table 1.
Ranking of the 10 VOCs that best distinguish the blends of the control and herbivory treatments as determined by the RF analysis. The compounds are listed in descending order based on decrease in variable importance (E).
| Rank | Compound | VOC class | E |
|---|---|---|---|
| 1 | 2-Methyl butanenitrile | OVOC | 110 |
| 2 | (E)-4,8-Dimethylnona-1,3,7-triene1 | HT | 81.52 |
| 3 | Benzyl nitrile | OVOC | 79.96 |
| 4 | 2-Butanone | OVOC | 76.04 |
| 5 | (E)-β-Ocimene1 | MT | 66.5 |
| 6 | (Z)-4,8-Dimethylnona-1,3,7-triene1 | HT | 63.01 |
| 7 | Linalool1 | MT | 61.33 |
| 8 | 1-Penten-3-ol | OVOC | 58.27 |
| 9 | (Z)-β-Ocimene1 | MT | 57.81 |
| 10 | Unidentified SQT 7 | SQT | 54.99 |
1Compound identification was verified by available pure standards. All compounds were released in significantly higher amounts from infested than control branches.
Herbivory effects on foliar NVSC contents
Our untargeted UHPLC–MS/MS analysis detected 235 NVSCs, of which 63 were annotated based on levels 1 and 2 identification according to community standards. Identified metabolites consisted of carboxylic acids and derivatives, flavonoids, organic oxygen compounds, prenol lipids and tannins (see Figure S2 and Table S5 available as Supplementary data at Tree Physiology Online). Random Forest (RF) could not separate the NVSC blends by the herbivory treatment before the defoliation period, whereas it could after the defoliation period (Figure 5A). Of the 10 most important NVSCs for the separation between the herbivory and control treatments, four belonged to the NVSC subclass of fatty acyl glucosides and one to the NVSC subclass of carbohydrates and carbohydrate conjugates, based on the feature-based molecular networking analysis (Table 2; see Figure S7 available as Supplementary data at Tree Physiology Online). The structure of the remaining five compounds was predicted using the spectral annotation software SIRIUS, which showed their chemical nature related to the class of terpene glycosides as well as 1-hydroxy-2-unsubstituted benzenoids (Table 2). The foliar contents of seven of these NVSCs increased, whereas three decreased, significantly in response to herbivory treatment after the defoliation period (Table 2).
Figure 5.
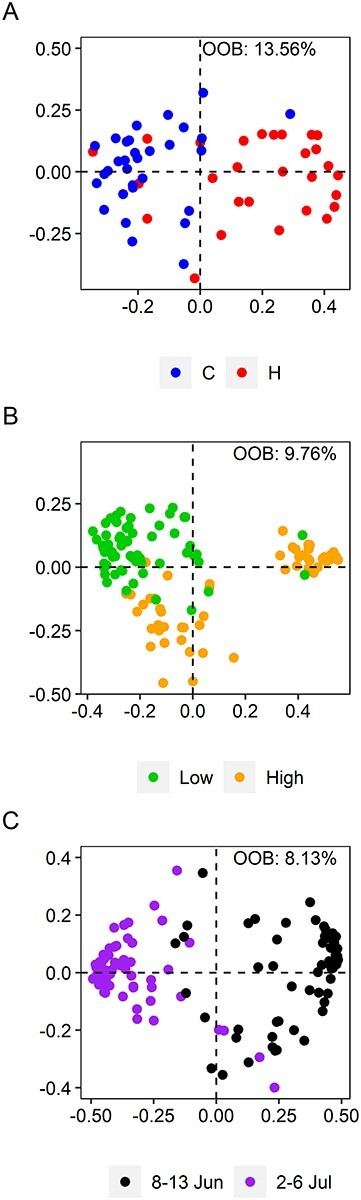
Metric multi-dimensional scaling plots of the results of the RF analyses on NVSCs. MDS plots of the proximity matrices showing the separation of the samples according to the NVSC blends, between the control and herbivory treatment after the defoliation period, i.e., 2–6 July, at the low elevation (A), between low and high elevations across both herbivory treatments and measurement times (B), and between measurement times across both herbivory treatments and elevations (C).
Table 2.
Ranking of the 10 NVSCs that best distinguish the blends of the control and herbivory treatments as determined by the RF analysis. The subclass of the individual compounds resulting from the feature-based molecular networking analysis is listed for each NVSC. The compounds are listed in descending order based on decrease in variable importance (E).
| Rank | ID | NVSC subclass | E |
|---|---|---|---|
| 1 | 226↑ | Fatty acyl glycosides | 137.51 |
| 2 | 81↑ | Terpene glycosides1 | 93.44 |
| 3 | 136↑ | NA | 77.57 |
| 4 | 133↑ | 1-Hydroxy-2-unsubstituted benzenoids1 | 74.69 |
| 5 | 84↓ | NA | 63.58 |
| 6 | 127↑ | Fatty acyl glycosides2 | 63.28 |
| 7 | 104↓ | Carbohydrates and carbohydrate conjugates | 61.24 |
| 8 | 97↑ | Fatty acyl glycosides | 57. 14 |
| 9 | 58↓ | Fatty acyl glycosides | 50.34 |
| 10 | 211↑ | Terpene glycosides1 | 48.03 |
Arrows beside ID numbers indicate the direction of the herbivory effect (↑: increase; ↓: decrease). The tandem mass spectra (MS/MS) of the 10 NVSCs are given in Figure S7, and details of the class, subclass and identity of each NVSC are shown in Table S5, both available as Supplementary data at Tree Physiology Online. NA: not available metabolite annotation/identification within the molecular network.
1Identified via SIRIUS.
2Tentatively identified as 7-epi-12-hydroxyjasmonic acid glucoside.
Differences in VOC emissions and foliar NVSC contents between elevations
The emissions of total VOCs, MTs, GLVs, OVOCs and SQTs were significantly higher at high elevation, compared with low elevation, in the early measurement (Figures 2B and 3; see Figure S5A available as Supplementary data at Tree Physiology Online). Furthermore, they were all having higher emissions at the high compared with low elevation when averaged across all measurement times. However, the HT emissions were higher at low elevation compared with high elevation when averaged across all measurement times. The VOC blend was best separated between the elevations during the early measurement (Figure 4B), whereas the NVSC blend was generally well separated between the elevations (Figure 5B).
Temporal variation of VOC emissions and foliar NVSC contents
VOC emissions varied substantially over the season, following the temporal variation of enclosure temperature and PPFD (Figures 2 and 3). Total VOC emissions were, indeed, positively correlated with the measured enclosure temperature (R2 = 0.209) and PPFD (R2 = 0.308; P < 0.001, Pearson’s correlation test). For example, the lowest constitutive and induced emissions of total VOCs and individual compounds were observed on 25–30 June when both the enclosure temperature and PPFD were very low (Figures 2 and 3). Generally, the RF analysis separated the VOC and NVSC blends between measurement times (Figures 4C and 5C).
Discussion
Herbivory effects on VOC emissions and foliar NVSC contents
The herbivory treatment, feeding of mountain birch foliage by winter moth, increased emission rates of total VOCs, most of the VOC classes and individual VOCs. MTs, HTs and GLVs responded most strongly to herbivory. Furthermore, the herbivory treatment also increased foliar NVSC contents. Overall, the herbivory effects were strongest towards the end of the defoliation period and stronger at low elevation compared with high elevation. This is most likely due to accumulative leaf damage nearer the end of the defoliation period and the higher herbivore pressure (i.e., greater number of larvae applied at the low elevation site). The VOC emission rates increase linearly with the severity of insect herbivory, as shown in many studies (as reviewed by Niinemets et al. 2013), including a recent field study on mountain birch (Rieksta et al. 2020).
The increase in herbivore-induced VOC emissions we observed in our study corresponds well with previous studies (Kesselmeier and Staudt 1999, Dicke et al. 2009, Tholl et al. 2011, Hartikainen et al. 2012, Ameye et al. 2018). However, only a few studies have investigated herbivore-induced VOC emission patterns in natural settings (Faiola and Taipale 2020), and even fewer located in the Subarctic Fennoscandia, as we did here (Mäntylä et al. 2008, Li et al. 2019, Rieksta et al. 2020). These studies report strong increases of isoprenoid VOCs and GLVs, in agreement with our study (Mäntylä et al. 2008, Li et al. 2019, Rieksta et al. 2020). The importance of these studies is further emphasized by in-field experiments generally indicating that herbivore-induced VOC emissions differ greatly between natural and controlled settings (Faiola and Taipale 2020). In our study, we advance the knowledge of herbivore-induced VOC emissions in natural settings by assessing the effects of herbivory throughout the defoliation period of the insects and the growing season, compared with previous studies commonly measuring for a couple of days to 1 week (Faiola and Taipale 2020).
Herbivore-induced VOCs have been suggested to function as a ‘cry for help’ by attracting herbivores’ natural enemies, though evidence from natural conditions remain rare (Turlings and Erb 2018). Our study detected several VOCs that are involved with indirect defenses against insect herbivores. For example, MTs such as linalool and ocimene, as well as the HTs, are known to be involved in indirect plant defenses by attracting natural enemies of herbivores (Tholl et al. 2011, McCormick et al. 2012, Turlings and Erb 2018, McCormick et al. 2019). Furthermore, the nine most important VOCs for the separation between treatments were compounds typically released for the attraction of natural enemies, such as the OVOCs, 2-methyl-butanenitrile and benzyl nitrile (McCormick et al. 2014), as well as 1-penten-3-ol and 2-butanone (Pinto-Zevallos et al. 2013). In mountain birch forest, earlier field studies have shown that birds could exploit VOCs released by mountain birch damaged by autumnal moth larvae to locate their prey (Mäntylä et al. 2008). In this regard, it is likely that in the present study, mountain birch altered VOC emissions in response to winter moth larval feeding to attract moths’ predators, such parasitoids and birds.
Birches are assumed to lack inner specific storage structures, meaning that many VOCs from birches are either released directly after de novo synthesis or from temporary non-specific storage (Ghirardo et al. 2010, Ormeño et al. 2011). The increased MT and HT emissions observed in our study are therefore most likely due to increased de novo synthesis in response to herbivory (Donath and Boland 1994, Pare and Tumlinson 1997, Ghirardo et al. 2010, Pinto-Zevallos et al. 2013, Ghirardo et al. 2020). Increased GLV emissions, on the other hand, are most likely due to GLVs bursting out upon foliar cell disruption caused by herbivore chewing (Heil 2009, Ameye et al. 2018).
We used an untargeted liquid chromatography–mass spectrometry (LC–MS) approach to investigate herbivory effects on the foliar NVSC contents and to identify compounds substantially affected by herbivory. Our untargeted LC–MS approach is distinctive compared to most other studies that only focus on a few selected NVSC classes, such as phenolics (Descombes et al. 2020). We investigated the 10 most important NVSCs driving the separation of the herbivory and control treatments. Four of the NVSCs were characterized as fatty acyl glycosides, of which one was specifically annotated as 7-epi-12-hydroxyjasmonic acid glucoside, whereas the remaining structures resembled jasmonic acid derivatives. These compounds are likely responding to the herbivory treatment as the jasmonic acid pathway is known to be strongly elicited upon leaf damage by tissue-chewing insects (Seto et al. 2011). One of the NVSCs was identified as a carbohydrate/carbohydrate conjugate whilst two of the compounds were identified as terpene glucosides. The terpene glucosides could potentially be glucosidically bound volatiles, facilitating the transportation and storage of VOCs (Song et al. 2018). In general, the glycosylation of NVSCs can be particularly advantageous for defense responses, as glycosylation can aid metabolite translocation, prevent phytotoxicity, or increase the toxicity to herbivores (Neilson et al. 2011). One compound was identified as a 1-hydroxy-2-unsubstituted benzenoid. Benzenoids have previously been shown to increase in response to herbivory (Lavergne et al. 2020). Volatile benzenoids can attract natural enemies of the herbivores (Liu et al. 2018, Lavergne et al. 2020).
In summary, the NVSC response to herbivory suggests that different classes of NVSCs, particularly jasmonic acid derivates, are substantially affected by herbivory. Furthermore, our results support the usefulness of untargeted LC–MS analyses for detecting NVSCs important for plant-herbivore interactions in mountain birch, beyond the often-studied phenolics. However, interpretation of the full chemical profile is challenging due to the high number of unknown compounds detected, leaving most of the data unexplored (Stavrianidi 2020). This opens future opportunities for investigating the metabolomic response of mountain birch and other forest trees to biotic and abiotic factors, and the development of new machine learning approaches based on mass spectral libraries would aid in such ventures (Blazenovic et al. 2018).
During the first campaign, it was noted that trees at the low elevation experienced aphid infestation. Even though aphids were removed from target branches before the experiment commenced, we cannot rule out potential legacy effects of previous aphid infestation on the target branches, nor the possibility of systemic effects caused by ongoing aphid feeding on adjacent branches of the measured trees. The co-occurrence of natural aphid infestation might interfere with the responses of VOCs and NVSCs to our experimental herbivory treatment and may thus have obscured the elevational effects on herbivore-induced responses, especially considering that aphid infestation has been shown to antagonize plant defense responses to attacks by chewing herbivores (Dicke et al. 2009). Future studies in natural settings are needed to assess to what extent local herbivore feeding triggers systemic responses, and how plant defense chemistry would respond to simultaneous or sequential attack by multiple herbivores of different feeding guilds (e.g., tissue-chewing versus phloem-sucking arthropods) as well as past insect and disease outbreaks.
Differences in VOC emissions and foliar NVSC contents between elevations
We compared the chemical defenses in mountain birch at two elevations, expecting that as temperature generally decreases with altitude, we could compare the elevations as two temperature treatments functioning as proxies for climate warming (Körner 2007, Moreira et al. 2018). Indeed, the air temperature was slightly higher at the low compared with high elevation primarily in the early growing season and in the autumn. However, the difference was too small to translate into differences in plant defense chemistry and we only observed weak elevation effects due to temperature.
The VOC emissions were generally higher at high elevation compared with low elevation, mainly driven by the high emissions during the early measurement. The NVSC blend was generally well separated between elevations, suggesting a difference in the foliar NVSC contents between low and high elevations. These results are most likely due to elevation related phenological differences; all leaves were unfolded at low elevation whereas they had just started to unfold at high elevation. As birch leaf chemistry changes rapidly in the early season (Riipi et al. 2002), the different developmental stages in the two elevations were naturally accompanied by large differences in specialized compounds. Our study suggests that unfolding young leaves of mountain birch may have stronger VOC emission capacities than older leaves. This could be due to a higher level of chemical defense in young, compared with mature, leaves (Bracho-Nunez et al. 2011, Vedel-Petersen et al. 2015). Previously, it has been suggested that leaf development regulates VOC emissions, with for example, decreased emissions of MTs and SQTs at leaf maturity (Bracho-Nunez et al. 2011). Vedel-Petersen et al. (2015) also recorded higher emissions of MTs and SQTs from dwarf birch in the early growing season that decreased over time. However, other elevation-related environmental variables (e.g., air humidity, soil moisture, solar radiation and wind) might also have played a role in shaping elevational variation in plant specialized compounds, as has been found in previous studies (Niinemets et al. 2010). To better elucidate elevational effects on plant chemistry and determine the main drivers behind them, future studies should use elevation gradients with contrasting thermal conditions, measure different environmental and biotic (e.g., foliar herbivory and disease, soil microbial diversity) factors, and statistically assess their relative importance. We also recommend assessing photosynthetic performance of the studied plants in order to better distinguish responses in plant physiology from those in secondary chemistry.
Temporal variations in VOC emissions and foliar NVSC contents
Temporal variations in VOC emissions largely followed the variation of the enclosure temperature and PPFD. This was expected because VOC emissions are known to be temperature- and light-dependent (Loreto and Fares 2013, Ghirardo et al. 2020). However, leaf developmental stage and variation in environmental factors, such as water and nutrient availability, could potentially have influenced temporal variations in VOC emissions (Hakola et al. 2001, Chen et al. 2020). Generally, the NVSC blend changed from the early measurement to mid measurement, suggesting a seasonal change in the foliar NVSC contents. This is in agreement with previous studies reporting changes in foliar NVSC contents with leaf development (Riipi et al. 2002, Haukioja 2003, Zidorn 2018). However, foliar NVSC contents also follow the variation of other factors, such as light, temperature, water and nutrient availability (Gobbo-Neto and Lopes 2007, Zidorn 2018).
Conclusions
We have, for the first time, assessed the combined effects of insect herbivory, elevation and season on mountain birch VOC emissions and NVSC production in a Fennoscandian birch forest. Our results show that feeding by winter moth larvae strongly alters birch VOC emissions and foliar NVSC contents, while elevation exerts little effect, which was most likely due to relatively similar temperatures between our low and high elevation sites. Furthermore, by using an untargeted LC–MS approach, we detected NVSC classes substantially affected by herbivory beyond the often-studied phenolics. The VOC emissions vary considerably over the growing season, mainly driven by seasonal variation in temperature and light intensity, whereas seasonal variations in foliar NVSC contents seems to be primarily driven by leaf development. Further studies employing distinct temperature differences along elevation gradients are needed to investigate how warming and herbivory interact to influence plant defense chemistry.
Data and materials availability
All VOC and NVSC data that support the findings of this study are available in Figshare (doi: 10.6084/m9.figshare.13370240) after the manuscript is accepted for publication.
The molecular networking job can be publicly accessed at https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=ac7bb0838b544d4c96f81a59aa9f5c32
The network annotation propagation job can be publicly accessed at https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=7f48f4c1f9804e6aaec5b7e4cade9b4c
Supplementary Material
Acknowledgments
We thank Annika Alsila and Rieke Lo Madsen for assistance in the field, Cleo Davie-Martin, Marios Vazakas, David Ian Pattison and Stefanie Petersen for assistance in the laboratory. Furthermore, we would like to thank COAT—(Climate-ecological Observatory for Arctic Tundra), who provided logistical support, lab and field facilities for the work.
Contributor Information
Ingvild Ryde, Terrestrial Ecology Section, Department of Biology, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen, Denmark; Section for Plant Biochemistry, Department of Plant and Environmental Sciences, University of Copenhagen, Thorvaldsensvej 40, DK-1871 Frederiksberg C, Denmark.
Tao Li, Terrestrial Ecology Section, Department of Biology, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen, Denmark; Center for Permafrost (CENPERM), Department of Geosciences and Natural Resource Management, University of Copenhagen, Øster Voldgade 10, DK-1350 Copenhagen K, Denmark.
Jolanta Rieksta, Terrestrial Ecology Section, Department of Biology, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen, Denmark; Center for Permafrost (CENPERM), Department of Geosciences and Natural Resource Management, University of Copenhagen, Øster Voldgade 10, DK-1350 Copenhagen K, Denmark.
Bruna M dos Santos, Section for Plant Biochemistry, Department of Plant and Environmental Sciences, University of Copenhagen, Thorvaldsensvej 40, DK-1871 Frederiksberg C, Denmark.
Elizabeth H J Neilson, Section for Plant Biochemistry, Department of Plant and Environmental Sciences, University of Copenhagen, Thorvaldsensvej 40, DK-1871 Frederiksberg C, Denmark.
Oliver Gericke, Section for Plant Biochemistry, Department of Plant and Environmental Sciences, University of Copenhagen, Thorvaldsensvej 40, DK-1871 Frederiksberg C, Denmark.
Jane U Jepsen, Department of Tromsø (NINA Tromsø), Norwegian Institute for Nature Research (NINA), Hjalmar Johansens Gate 14, NO-9296 Tromsø, Norway.
Louise R H Bork, Terrestrial Ecology Section, Department of Biology, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen, Denmark.
Hildur S Holm, Terrestrial Ecology Section, Department of Biology, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen, Denmark.
Riikka Rinnan, Terrestrial Ecology Section, Department of Biology, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen, Denmark; Center for Permafrost (CENPERM), Department of Geosciences and Natural Resource Management, University of Copenhagen, Øster Voldgade 10, DK-1350 Copenhagen K, Denmark.
Conflict of interest
None declared.
Funding
This work was financed by the European Research Council (ERC) under The European Union’s Horizon 2020 research and innovation programme (771012 to R.R.); the Danish National Research Foundation (CENPERM DNRF100); the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie (751684 to T.L.); the Research Council of Norway (244454/E10 to J.U.J.); a Novo Nordisk Emerging Investigator Grant (0054890 to E.H.J.N.); a VILLUM Foundation Young Investigator Grant (13167 to E.H.J.N.); and a Sapere Aude Postdoctoral Fellowship (6111-00379B to E.H.J.N.).
Authors’ contributions
T.L. and R.R. designed the experiment. T.L., J.R. and R.R. designed the methods for VOC analysis. E.H.J.N. and B.M.S. designed the methods for NVSC analysis. OG conducted the untargeted UHPLC–MS/MS spectral data processing as well as subsequent molecular networking and SIRIUS-based dereplication pipeline. JUJ provided the experimental sites and laboratory resources at the Norwegian Institute for Nature Research (NINA) in Tromsø, and collected the insects. T.L., I.R., J.R., L.R.H.B. and H.S.H. executed the experiment and collected the data. I.R. and T.L. performed data analyses and wrote the manuscript with contributions from all authors.
References
- Ameye M, Allmann S, Verwaeren J, Smagghe G, Haesaert G, Schuurink RC, Audenaert K (2018) Green leaf volatile production by plants: a meta-analysis. New Phytol 220:666–683. [DOI] [PubMed] [Google Scholar]
- Ammunet T, Bylund H, Jepsen JU (2015) Northern geometrids and climate change: from abiotic factors to trophic interactions. In: Bjorkman C, Niemela P (eds) Climate change and insect pests. CABI Publishing, Wallingford, pp 235–247. [Google Scholar]
- Bale JS, Masters GJ, Hodkinson ID et al. (2002) Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Chang Biol 8:1–16. [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. [Google Scholar]
- Blazenovic I, Kind T, Ji J, Fiehn O (2018) Software tools and approaches for compound identification of LC-MS/MS data in metabolomics. Metabolites 8:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böcker S, Dührkop K (2016) Fragmentation trees reloaded. J Chem 8:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracho-Nunez A, Welter S, Staudt M, Kesselmeier J (2011) Plant-specific volatile organic compound emission rates from young and mature leaves of Mediterranean vegetation. J Geophys Res Atmos 116:1–13. [Google Scholar]
- Breiman L (2001) Random forests. Mach Learn 45:5–32. [Google Scholar]
- Chen JG, Tang J, Yu XX (2020) Environmental and physiological controls on diurnal and seasonal patterns of biogenic volatile organic compound emissions from five dominant woody species under field conditions. Environ Pollut 259:1–12. [DOI] [PubMed] [Google Scholar]
- Close DC, McArthur C (2002) Rethinking the role of many plant phenolics - protection from photodamage not herbivores? Oikos 99:166–172. [Google Scholar]
- Descombes P, Kergunteuil A, Glauser G, Rasmann S, Pellissier L (2020) Plant physical and chemical traits associated with herbivory in situ and under a warming treatment. J Ecol 108:733–749. [Google Scholar]
- Dicke M, van Loon JJA, Soler R (2009) Chemical complexity of volatiles from plants induced by multiple attack. Nat Chem Biol 5:317–324. [DOI] [PubMed] [Google Scholar]
- Donath J, Boland W (1994) Biosynthesis of acyclic homoterpenes in higher plants parallels steroid-hormone metabolism. J Plant Physiol 143:473–478. [Google Scholar]
- dos Santos BM, Zibrandtsen JFS, Gunbilig D, Sorensen M, Cozzi F, Boughton BA, Heskes AM, Neilson EHJ (2019) Quantification and localization of formylated phloroglucinol compounds (FPCs) in Eucalyptus species. Front Plant Sci 10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dührkop K, Fleischauer M, Ludwig M, Aksenov AA, Melnik AV, Meusel M, Dorrestein MC, Rousu J, Böckeret S (2019) SIRIUS 4: a rapid tool for turning tandem mass spectra into metabolite structure information. Nat Methods 16:299–302. [DOI] [PubMed] [Google Scholar]
- Dührkop K, Shen H, Meusel M, Rousu J, Böcker S (2015) Searching molecular structure databases with tandem mass spectra using CSI: FingerID. Proc Natl Acad Sci USA 112:12580–12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dührkop K, Nothias LF, Fleischauer M et al. (2020) Systematic classification of unknown metabolites using high-resolution fragmentation mass spectra. Nat Biotechnol 1–10. doi: 10.1038/s41587-020-0740-8 [DOI] [PubMed] [Google Scholar]
- Faiola C, Taipale D (2020) Impact of insect herbivory on plant stress volatile emissions from trees: a synthesis of quantitative measurements and recommendations for future research. Atmos Environ X 5:1–17. [Google Scholar]
- Gericke O, Fowler RM, Heskes AM et al. (2020) Navigating through chemical space and evolutionary time across the Australian continent in plant genus Eremophila. bioRxiv 1–121. doi: 10.1101/2020.11.02.364471; preprint, not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardo A, Koch K, Taipale R, Zimmer I, Schnitzler JP, Rinne J (2010) Determination of de novo and pool emissions of terpenes from four common boreal/alpine trees by 13CO2 labelling and PTR-MS analysis. Plant Cell Environ 33:781–792. [DOI] [PubMed] [Google Scholar]
- Ghirardo A, Lindstein F, Koch K, et al. (2020) Origin of volatile organic compound emissions from subarctic tundra under global warming. Glob Chang Biol 26:1908–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbo-Neto L, Lopes NP (2007) Medicinal plants: factors of influence on the content of secondary metabolites. Quim Nova 30:374–381. [Google Scholar]
- Hakola H, Laurila T, Lindfors V, Hellen H, Gaman A, Rinne J (2001) Variation of the VOC emission rates of birch species during the growing season. Boreal Environ Res 6:237–249. [Google Scholar]
- Hammond-Kosack KE, Jones JDG (2015) Responses to plant pathogens. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. John Wiley and Sons Ltd, West Sussex, pp 984–1050. [Google Scholar]
- Hare JD (2010) Ontogeny and season constrain the production of herbivore-inducible plant volatiles in the field. J Chem Ecol 36:1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartikainen K, Riikonen J, Nerg A-M, et al. (2012) Impact of elevated temperature and ozone on the emission of volatile organic compounds and gas exchange of silver birch (Betula pendula Roth). Environ Exp Bot 84:33–43. [Google Scholar]
- Haukioja E (2003) Putting the insect into the birch-insect interaction. Oecologia 136:161–168. [DOI] [PubMed] [Google Scholar]
- Haukioja E (2005) Plant defenses and population fluctuations of forest defoliators: mechanism-based scenarios. Ann Zool Fenn 42:313–325. [Google Scholar]
- Heil M (2009) Damaged-self recognition in plant herbivore defence. Trends Plant Sci 14:356–363. [DOI] [PubMed] [Google Scholar]
- Holopainen JK, Virjamo V, Ghimire RP, Blande JD, Julkunen-Tiitto R, Kivimaenpaa M (2018) Climate change effects on secondary compounds of forest trees in the Northern Hemisphere. Front Plant Sci 9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Jander G (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59:41–66. [DOI] [PubMed] [Google Scholar]
- IPCC (2014) Climate change 2014: Synthesis report (eds Pachauri RK et al.). University Press, Cambridge. [Google Scholar]
- Johnsen LG, Skou PB, Khakimov B, Bro R (2017) Gas chromatography - mass spectrometry data processing made easy. J Chromatogr A 1503:57–64. [DOI] [PubMed] [Google Scholar]
- Kesselmeier J, Staudt M (1999) Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J Atmos Chem 33:23–88. [Google Scholar]
- Körner C (2007) The use of 'altitude' in ecological research. Trends Ecol Evol 22:569–574. [DOI] [PubMed] [Google Scholar]
- Kutchan TM, Gershenzon J, Møller BL, Gang DR (2015) Natural products. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. John Wiley and Sons Ltd, West Sussex, pp 1132–1206. [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest Package: tests in linear mixed effects models. J Stat Softw 82:1–26. [Google Scholar]
- Lavergne FD, Broeckling CD, Brown KJ, et al. (2020) Differential stem proteomics and metabolomics profiles for four wheat cultivars in response to the insect pest wheat stem sawfly. J Proteome Res 19:1037–1051. [DOI] [PubMed] [Google Scholar]
- Li T, Holst T, Michelsen A, Rinnan R (2019) Amplification of plant volatile defence against insect herbivory in a warming Arctic tundra. Nat Plants 5:568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw A, Wiener M (2002) Classification and regression by randomForest. R News 2:18–22. [Google Scholar]
- Liu B, Kaurilind E, Jiang YF, Niinemets U (2018) Methyl salicylate differently affects benzenoid and terpenoid volatile emissions in Betula pendula. Tree Physiol 38:1513–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Fares S (2013) Biogenic volatile organic compounds and their impacts on biosphere-atmosphere interactions. In: Matyssek R, Clarke N, Cudlin P, Mikkelsen TN, Tuovinen JP, Wieser G, Paoletti E (eds) Climate change, air pollution and global challenges: understanding and perspectives from forest research. Elsevier Science Bv, Amsterdam, pp 57–75. [Google Scholar]
- Loreto F, Schnitzler JP (2010) Abiotic stresses and induced BVOCs. Trends Plant Sci 15:154–166. [DOI] [PubMed] [Google Scholar]
- Mäntylä E, Alessio GA, Blande JD, Heijari J, Holopainen JK, Laaksonen T, Piirtola P, Klemola T (2008) From plants to birds: higher avan predation rates in trees responding to insect herbivory. PLoS One 3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick AC, Unsicker SB, Gershenzon J (2012) The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci 17:303–310. [DOI] [PubMed] [Google Scholar]
- McCormick AC, Irmisch S, Reinecke A, et al. (2014) Herbivore-induced volatile emission in black poplar: regulation and role in attracting herbivore enemies. Plant Cell Environ 37:1909–1923. [DOI] [PubMed] [Google Scholar]
- McCormick AC, Irmisch S, Boeckler GA, Gershenzon J, Kollner TG, Unsicker SB (2019) Herbivore-induced volatile emission from old-growth black poplar trees under field conditions. Sci Rep 9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira X, Petry WK, Mooney KA, Rasmann S, Abdala-Roberts L (2018) Elevational gradients in plant defences and insect herbivory: recent advances in the field and prospects for future research. Ecography 41:1485–1496. [Google Scholar]
- Neilson EH, Goodger JQD, Motawia MS, Bjarnholt N, Frisch T, Olsen CE, Moller BL, Woodrow IE (2011) Phenylalanine derived cyanogenic diglucosides from Eucalyptus camphora and their abundances in relation to ontogeny and tissue type. Phytochemistry 72:2325–2334. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Arneth A, Kuhn U, Monson RK, Peñuelas J, Staudt M (2010) The emission factor of volatile isoprenoids: stress, acclimation, and developmental responses. Biogeosciences 7:2203–2223. [Google Scholar]
- Niinemets Ü, Kännaste A, Copolovici L (2013) Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front Plant Sci 4:1–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIST (2014) Standard reference database 1A - NIST thermodynamic and transport properties of pure fluids-NIST pure fluids. National Institute of Standards and Technology, Gaitersburg, Maryland. [Google Scholar]
- Opala M, Migala K, Owczarek P (2016) Two centuries-long dendroclimatic reconstruction based on low Arctic Betula pubescens from Tromso region, northern Norway. Pol Polar Res 37:457–476. [Google Scholar]
- Ormeño E, Goldstein A, Niinemets U (2011) Extracting and trapping biogenic volatile organic compounds stored in plant species. Trends Analyt Chem 30:978–989. [Google Scholar]
- Ortega J, Helmig D (2008) Approaches for quantifying reactive and low-volatility biogenic organic compound emissions by vegetation enclosure techniques - part A. Chemosphere 72:343–364. [DOI] [PubMed] [Google Scholar]
- Ossipov V, Klemola T, Ruohomaki K, Salminen JP (2014) Effects of three years' increase in density of the geometrid Epirrita autumnata on the change in metabolome of mountain birch trees (Betula pubescens ssp czerepanovii). Chemoecology 24:201–214. [Google Scholar]
- Pare PW, Tumlinson JH (1997) De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol 114:1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñuelas J, Llusià J (1999) Seasonal emission of monoterpenes by the Mediterranean tree Quercus ilex in field conditions: relations with photosynthetic rates, temperature and volatility. Physiol Plant 105:641–647. [Google Scholar]
- Peñuelas J, Staudt M (2010) BVOCs and global change. Trends Plant Sci 15:133–144. [DOI] [PubMed] [Google Scholar]
- Pepi AA, Vindstad OPL, Malin EK, Jepsen JU (2017) Elevationally biased avian predation as a contributor to the spatial distribution of geometrid moth outbreaks in subarctic mountain birch forest. Ecol Entomol 42:430–438. [Google Scholar]
- Pinto-Zevallos DM, Hellen H, Hakola H, van Nouhuys S, Holopainen JK (2013) Induced defenses of Veronica spicata: variability in herbivore-induced volatile organic compounds. Phytochem Lett 6:653–656. [Google Scholar]
- Pluskal T, Castillo S, Villar-Briones A, Orešič M (2010) MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform 11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at: https://www.R-project.org/. [Google Scholar]
- Ranganathan Y, Borges RM (2010) Reducing the babel in plant volatile communication: using the forest to see the trees. Plant Biol 12:735–742. [DOI] [PubMed] [Google Scholar]
- Rieksta J, Li T, Junker RR, Jepsen JU, Ryde I, Rinnan R (2020) Insect herbivory strongly modifies mountain birch volatile emissions. Front Plant Sci 11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riipi M, Ossipov V, Lempa K, Haukioja E, Koricheva J, Ossipova S, Pihlaja K (2002) Seasonal changes in birch leaf chemistry: are there trade-offs between leaf growth, and accumulation of phenolics? Oecologia 130:380–390. [DOI] [PubMed] [Google Scholar]
- Rinnan R, Steinke M, McGenity T, Loreto F (2014) Plant volatiles in extreme terrestrial and marine environments. Plant Cell Environ 37:1776–1789. [DOI] [PubMed] [Google Scholar]
- Russell L (2019) emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.4.2. https://CRAN.R-project.org/package=emmeans. [Google Scholar]
- Schymanski EL, Jeon J, Gulde R, Fenner K, Ruff M, Singer HP, Hollender J (2014) Identifying small molecules via high resolution mass spectrometry: communicating confidence. Environ Sci Technol 48:2097–2098. [DOI] [PubMed] [Google Scholar]
- Seto Y, Hamada S, Ito H, Masuta C, Matsui H, Nabeta K, Matsuura H (2011) Tobacco salicylic acid glucosyltransferase is active toward tuberonic acid (12-hydroxyjasmonic acid) and is induced by mechanical wounding stress. Biosci Biotechnol Biochem 75:2316–2320. [DOI] [PubMed] [Google Scholar]
- Song CK, Hartl K, McGraphery K, Hoffmann T, Schwab W (2018) Attractive but toxic: emerging roles of glycosidically bound volatiles and glycosyltransferases involved in their formation. Mol Plant 11:1225–1236. [DOI] [PubMed] [Google Scholar]
- Stavrianidi A (2020) A classification of liquid chromatography mass spectrometry techniques for evaluation of chemical composition and quality control of traditional medicines. J Chromatogr A 1609:1–25. [DOI] [PubMed] [Google Scholar]
- Tenow O, Bylund H (2000) Recovery of a Betula pubescens forest in northern Sweden after severe defoliation by Epirrita autumnata. J Veg Sci 11:855–862. [Google Scholar]
- Tholl D, Sohrabi R, Huh JH, Lee S (2011) The biochemistry of homoterpenes - common constituents of floral and herbivore-induced plant volatile bouquets. Phytochemistry 72:1635–1646. [DOI] [PubMed] [Google Scholar]
- Trowbridge AM, Stoy PC (2013) BVOC-mediated plant-herbivore interactions. In: Niinemets Ü, Monson RK (eds) Biology, controls and models of tree volatile organic compound emissions. Springer, New York, NY, pp 21–46. [Google Scholar]
- Trowbridge AM, Daly RW, Helmig D, Stoy PC, Monson RK (2014) Herbivory and climate interact serially to control monoterpene emissions from pinyon pine forests. Ecology 95:1591–1603. [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Erb M (2018) Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu Rev Entomol 63:433–452. [DOI] [PubMed] [Google Scholar]
- Vedel-Petersen I, Schollert M, Nymand J, Rinnan R (2015) Volatile organic compound emission profiles of four common arctic plants. Atmos Environ 120:117–126. [Google Scholar]
- Wang L, Wu JQ (2013) The essential role of jasmonic acid in plant-herbivore interactions - using the wild tobacco Nicotiana attenuata as a model. J Genet Genomics 40:597–606. [DOI] [PubMed] [Google Scholar]
- Wielgolaski F (2005) History and environment of the Nordic mountain birch. In: Caldwell MM et al. (eds). Plant ecology, herbivory, and human impact in Nordic Mountain Birch Forest, Springer, Berlin, Heidelberg, pp 3–18. [Google Scholar]
- Zidorn C (2018) Seasonal variation of natural products in European trees. Phytochem Rev 17:923–935. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


