Abstract
Despite significant morbidity among infants with single ventricle heart disease (SVHD), clinical monitoring is limited by poor understanding of the underlying pathobiology. Proteomics can identify novel biomarkers and important pathways in complex disease. No prior study has evaluated whether the proteome of SVHD infants differs from healthy controls, how it shifts after stage 2 palliation, or whether differences can predict post-operative outcomes. We present a prospective cohort study of cardiovascular proteomic phenotyping in infants with SVHD undergoing stage 2 palliation. Twenty-nine pre-stage-2 SVHD infants and 25 healthy controls were enrolled. Outcomes included post-operative hypoxemia and endotracheal intubation time (ETT). Serum samples were drawn pre-operatively (systemic and pulmonary vein) and at 24 hours post-operation. Targeted cardiovascular proteomic analysis included 184 proteins. Partial least squares discriminant analysis (PLS-DA) distinguished cases from controls (Accuracy=0.98, R2=0.93, Q2=0.81) with decreased inflammatory mediators and increased modulators of vascular tone. PLS-DA also distinguished cases pre-operation vs. post-operation (Accuracy=0.98, R2=0.99, Q2=0.92) with post-operative increase in both inflammatory and vascular tone mediators. Pre-operation pulmonary vein tissue inhibitor of metalloproteinase-1 (TIMP1, 1.8x-fold, p=1.6x10−4) and nidogen-1 (1.5x-fold, p=1.7x10−4) were higher in subjects with longer ETT. Post-operation matrix metalloproteinase (MMP) 7 levels were higher in subjects with greater post-operative hypoxemia (1.5x-fold, p=1.97x10−5). Proteomic analysis identifies significant changes among SVHD infants pre- and post-stage 2, and healthy controls. TIMP-1, nidogen-1, and MMP7 levels are higher in SVHD cases with greater morbidity suggesting an important role for regulation of extracellular matrix production. Proteomic profiling may identify high-risk SVHD infants.
Keywords: Matrix Metalloproteinase, Cardiopulmonary Bypass, Bidirectional Glenn, Angiotensin Converting Enzyme 2, Adrenomedullin
Introduction
Congenital heart disease (CHD) is the most common severe birth defect in the United States, with estimated incidence of 4-10 per 1000 live births.1 A severe subset of CHD where subjects are born with only one pumping chamber, single ventricle heart disease (SVHD) represents between 2 and 8 in 10,000 live births and is fatal without intervention.1 Although there is no cure, surgical palliation for SVHD allows survival into adulthood for many patients and typically is completed in three stages. After completion of staged palliation, patients have pulsatile systemic arterial blood flow driven by the single ventricle but passive flow directly into the lungs without an intervening pump.2 From the first week of life (after stage 1 palliation for most patients) until the Stage 2 operation, subjects are referred to as being “inter-stage”; during this period they have an unobstructed pathway from the single ventricle to the body and a pressure-limited source of pulmonary blood flow, commonly a shunt between the aorta or the right ventricle and the pulmonary arteries. The second of the three typical operations, the superior cavo-pulmonary anastomosis (Stage 2-bidirectional Glenn or hemi-Fontan), is most often performed between 4-6 months of age.2 This operation is unique in that a patient’s mechanism of blood delivery to the lungs is surgically converted from an active process driven by ventricular systole during inter-stage to a passive process via a direct connection of the superior vena cava to the pulmonary arteries. This transition makes the peri-stage 2 period the optimal time to evaluate both pre-operative markers of inter-stage pulmonary vascular growth the post-operative sufficiency of the pulmonary vasculature to accept passive blood flow.
For many children with SVHD, pulmonary vascular inadequacy results in morbidity before and after Stage 2 palliation.3 The death or heart transplantation rate for SVHD patients is ~30% in the first year of life, including 12% mortality prior to Stage 2 palliation.4,5 Of those infants who undergo Stage 2, up to 25% experience complications in the immediate post-operative period that are directly linked to impaired pulmonary blood flow including severe hypoxemia, respiratory failure, and persistent pleural effusions.3,6 Diagnosis and treatment of pathologic pulmonary vascular development is limited due to poor understanding of the underlying mechanisms. Current monitoring includes serial oxygen saturation measurements, echocardiograms, cardiac MRI/CT, and cardiac catheterizations.7-10 There are no validated biomarkers to identify subjects in this population at risk for pathologic vascular development.
Proteomic analyses represent a new systems biology approach to identify novel biomarkers and important pathways in complex disease.11 Prior studies have demonstrated proteomic fingerprints with prognostic implications in patients with idiopathic pulmonary fibrosis, congestive heart failure, and ventricular septal defects.12-15 No prior study has evaluated the proteome of infants with SVHD undergoing staged palliation or its association with post-Stage 2 outcomes.
Here we present a prospective, cohort study of targeted cardiovascular proteomic phenotyping in infants with SVHD undergoing Stage 2 palliation. We hypothesized that SVHD infants would have a distinct circulating pre-operative proteomic fingerprint compared to similar age healthy controls, that the circulating proteome would shift immediately after Stage 2 palliation, and that both the measured proteome and specific individual proteins would be useful to identify SVHD patients at risk for post-Stage 2 complications associated with pulmonary vascular inadequacy.
Materials and Methods
The Colorado Multiple Institution Review Board approved this study. Written informed consent was obtained from the study subjects’ parents in all cases.
Subjects
We prospectively enrolled consecutive subjects age 31 days to 2 years at Children’s Hospital Colorado with SVHD either undergoing pre-Stage 2 catheterization or Stage 2 palliation without plans for cardiac catheterization. Subjects who underwent Stage 2 without sample collection at cardiac catheterization did so because 1) cardiac catheterization was performed prior to the research team having an opportunity to obtain informed consent or 2) catheterization was deemed not clinically required by the clinical team. Stage 2 palliation included any form of superior cavo-pulmonary anastomosis (Glenn or Hemi-Fontan operations) regardless of whether a patient had a prior Stage 1 palliation. Patients with a persistent, additional pulsatile source of pulmonary blood flow after Stage 2 (so-called 1.5 ventricle repair) were excluded.
Control subject candidates were identified from the surgical schedule at Children’s Hospital Colorado. Inclusion criteria were patients >4 kg aged 3-12 months undergoing anesthesia for elective, non-cardiac procedures with clinical need for IV access. Control subjects were excluded if they had any known cardiac, pulmonary, infectious, or genetic abnormalities.
Clinical Data
Clinical information was extracted from the electronic medical record (Epic Systems, Verona, WI). Post-operative oxygen saturation measurements were extracted at 1-minute intervals from the BedMaster hemodynamic monitoring system in the cardiac intensive care unit (CICU) (Anandic Medical Systems, Feuerthalen, Switzerland). Study data were collected and managed using REDCap electronic data capture tools hosted at University of Colorado.
Pre-specified clinical variables were identified to evaluate the relationship between proteomic biomarkers of interest and subjects with more or less favorable clinical outcomes. The primary clinical variable of interest was percent of time in the first 24 post-operative hours with clinically significant hypoxemia, defined as an oxygen saturation below 70% (24h Low Sat%). Secondary variables included endotracheal intubation time (ETT), chest tube days, and volume of chest tube drainage.
Sample Collection
All pre-operative samples were obtained under general anesthesia. For SVHD subjects enrolled at the time of pre-Stage 2 catheterization, systemic venous samples were obtained prior to any procedural interventions. To evaluate whether key proteins are affected by passing through the lung capillary network, an additional pulmonary venous sample was collected during catheterization for comparison to the systemic venous samples. For subjects enrolled at other times, a systemic venous sample was obtained on the day of Stage 2 palliation before initiation of cardiopulmonary bypass. Post-operative systemic venous samples were obtained 24 hours after arrival in the CICU. Control subject samples were obtained from a systemic vein after induction of anesthesia at the time of IV placement.
Protein Analysis
All blood samples were processed for serum at the time of collection and stored at −80C for batch analysis. Samples underwent a targeted cardiovascular proteomics analysis using the Olink Cardiometabolic and Cardiovascular II panels (Olink Proteomics, Uppsala Sweden) at their laboratory in Boston, MA.16 The list of measured proteins can be found on OLink’s website (www.OLink.com) as updated on 4/14/2020. Each Olink kit is a proximity extension assay that measures the relative abundance of 92 proteins (92*2 panels=184 total proteins). For each protein target, oligonucleotide-labeled antibody pairs bind to specific epitopes on the protein surface. Complementary oligonucleotide sequences give rise to DNA reporter sequences, which are then quantified using real-time PCR. Mean intra- and inter-assay coefficients of variation are 8 and 12%, respectively. Log2 scaled normalized protein expression values were adjusted by a negative control sample. Higher expression values correspond to higher protein levels but are not an absolute quantification of protein concentrations.
Statistical Analysis
Demographic and clinical variables were summarized using descriptive statistics as indicated by the distribution of the data. Wilcoxon rank sum and Spearman correlation testing were used to compare continuous variables and chi squared testing was used for categorical variables. JMP pro v.14.1.0 was used for descriptive analysis. P<0.05 was considered statistically significant.
Proteomic analysis was performed using Metaboanalyst 4.0 (www.metaboanalyst.ca). Prior to analysis, relative protein concentrations (previously log transformed) were auto scaled (mean centered and divided by the square root of the standard deviation of each variable). Partial least squares-discriminant analysis (PLS-DA) was performed to assess for capacity to distinguish between specified groups of interest based on global changes in the proteomic phenotype. Accuracy, R2 (goodness of fit), and Q2 (consistency on cross-validation) are reported for each model. Variable importance in projection (VIP) scores were used to identify the top proteins driving variation between groups. After log transformation to achieve a normal distribution, Student’s t and paired t testing were performed. Raw p-values are reported along with two methods of correction for multiple comparisons: Bonferroni and the False Discovery Rate (FDR). Findings were classified as highly statistically significant if they met the conservative Bonferroni correction threshold (p<0.00027) and of intermediate statistical significance they met the FDR cutoff of <0.05 but not the Bonferroni threshold.
Results
Study Population
Twenty-nine cases with SVHD and twenty-five similar age healthy controls were enrolled. Pre-operative systemic vein samples were available for all cases and controls. A pre-operative pulmonary vein sample was collected for 19 cases. One subject was deemed to not be a good candidate for continued single ventricle palliation and underwent heart transplantation instead of Stage 2 palliation. One subject ultimately underwent complete repair with a two-ventricle circulation. Twenty-seven cases were therefore included in the post-operative analysis. Demographics and clinical characteristics are presented in table 1.
Table 1.
Demographics
| SVHD Cases (n=29) |
Controls (n=25) | P Value | ||
|---|---|---|---|---|
| Sex (F) | 15 (52%) | 8 (32%) | 0.224 | |
| Weight [kg] | 5.5 (5.2-6.2) | 7.9 (7.1-8.8) | 0.001 | |
| Age [m] | 4.5 (3.9-5.4) | 6.8 (6.0-8.7) | 0.001 | |
| Diagnosis | HLHS | 17 (59%) | ||
| Unbalanced AVSD | 4 (14%) | |||
| DORV | 5 (17%) | |||
| DILV | 2 (7%) | |||
| TA | 1 (3%) | |||
| Stage 1 | Norwood | 17 (59%) | ||
| PA Band | 4 (14%) | |||
| Shunt | 5 (17%) | |||
| None | 3 (10%) | |||
| Pre-Stage 2 Cath | Mean PA Pressure [mmHg] | 12 (11-15) | ||
| PVRi [units*m2] | 1.6 (1.3-2.3) | |||
| Qp/Qs | 1.3 (0.9-1.8) | |||
| O2 Saturation [%] | 75% (73-80) | |||
| Cath-Surgery Delay [d] | 20 (12-43) | |||
| Post Stage 2 Outcomes | CPB time [m] | 138 (106-176) | ||
| Chest tube duration [d] | 3 (2-4) | |||
| Chest tube drainage [mL] | 153 (90-163) | |||
| ET intubation duration [h] | 18 (9-40) | |||
| Mean saturation first 24 hours [%] | 79 (76-80) | |||
| Percent of Time below 70% SpO2 first 24 hours [%] | 4.3 (1.7-9.0) | |||
| Discharge SpO2 [%] | 83 (80-86) | |||
| Discharge ETRA or PDE 5i [n] | 12 (41%) | |||
| Discharge oxygen therapy [n] | 29 (100%) |
Data represented as median (IQR) or n (%). HLHS=hypoplastic left heart syndrome, AVSD=atrioventricular septal defect, DORV=double outlet right ventricle, DILV=double inlet left ventricle, TA=tricuspid atresia, PA=pulmonary artery, PVRi=indexed pulmonary vascular resistance, CPB=cardio pulmonary bypass, ET=endotracheal, ETRA=endothelin receptor antagonist, PDE 5i=phosphodiesterase type 5 inhibitor.
Immediate post-Stage 2 Clinical Outcomes
The study population (n=27) experienced a moderate degree of post-operative morbidity. Half of subjects spent >50% of the first 24 post-operative hours with systemic oxygen saturation (SpO2) less than 80%. The median 24h Low Sat% was 4.3% (range 0.1%-60.7%; inter-quartile range 1.7%-9.0%). Median post-operative endotracheal intubation and chest tube duration times were 18 hours and 3 days, respectively. No patients required an emergent return to the operating room and all patients survived to hospital discharge. All patients received oxygen therapy throughout their post-Stage 2 course and were discharged home on supplemental oxygen. Twelve patients were additionally treated with pulmonary vasodilators, primarily phosphodiesterase type 5 inhibitors.
Pre-Operative Proteome in SVHD Cases and Controls
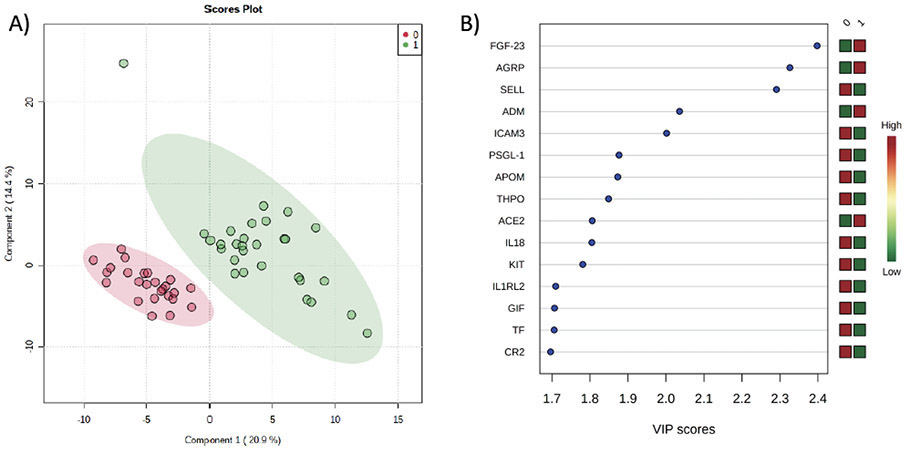
We first analyzed whether there were differences in the targeted cardiovascular proteome between SVHD cases (n=29) and controls (figure 1). PLS-DA readily discriminated between cases and controls based on their proteomic pattern (Accuracy=0.98, R2=0.93, Q2=0.81). The 15 markers with greatest effect on the proteomic phenotype between the groups by VIP analysis are shown in figure 1b. On single variable testing without correction for multiple comparisons, p<0.05 was observed for 90/184 tested protein markers with 73 reaching intermediate significance by FDR (supplemental table 1). After Bonferroni correction, 32 tested protein markers showed a highly significant difference (table 2). The majority of these proteins can be categorized as related to modulation of inflammation, cell metabolism, neurohormonal modulators of vascular tone, or coagulation. Proteins related to inflammation, cell metabolism, and coagulation were most often decreased in SVHD cases compared to controls while those related to vascular tone were more often increased.
Figure 1:
Comparison of the Proteomic Signature between Inter-Stage SVHD Cases (1) and Controls (0). A) Partial least squares-discriminant analysis demonstrates a significant global shift in the proteome between controls (red circles) and SVHD cases pre-operatively (green circles). Accuracy=0.98, R2=0.93, Q2=0.81. B) Variable importance projection scores for the 15 most important proteins to distinguish the proteome of SVHD cases and controls. FGF=fibroblast growth factor, AGRP=Agouti-related protein, SELL=L-selectin, ADM=adrenomedullin, ICAM=intercellular adhesion molecule, PSGL=P-selectin glycoprotein ligand, APOM=apolipoprotein M, THPO=thrombopoietin, ACE=angiotensin converting enzyme, IL=interleukin, IL1 RL2=IL1 receptor ligand 2, GIF=gastric intrinsic factor, TF=tissue factor, CR=complement receptor.
Table 2.
Proteins with Different Concentration (corrected p<0.05) Between Pre-Op SVHD Cases and Controls
| Protein | Fold Change | Raw P Value | Category |
|---|---|---|---|
| PSGL-1 | −1.3x | 1.70E-07 | Inflammation |
| CD46 | −1.4x | 8.62E-05 | Inflammation |
| IL1RL2 | −1.5x | 4.70E-06 | Inflammation |
| TNFR SF13B | −1.5x | 1.74E-04 | Inflammation |
| ICAM3 | −1.6x | 1.30E-08 | Inflammation |
| CD84 | −1.7x | 1.15E-05 | Inflammation |
| THPO | −1.7x | 2.87E-07 | Inflammation |
| CR2 | −1.8x | 3.91E-06 | Inflammation |
| L-Selectin | −1.9x | 7.62E-12 | Inflammation |
| IL18 | −1.9x | 9.07E-07 | Inflammation |
| CD40-L | −3.0x | 5.61E-05 | Inflammation |
| AGRP | +2.4x | 2.55E-12 | Cell Metabolism |
| Sortilin 1 | −1.3x | 2.06E-04 | Cell Metabolism |
| CA3 | −1.4x | 1.18E-04 | Cell Metabolism |
| APOM | −1.6x | 1.80E-07 | Cell Metabolism |
| GIF | −2.1x | 4.61E-06 | Cell Metabolism |
| BNP | +3.2x | 7.28E-06 | Vascular Tone |
| ACE2 | +1.9x | 9.19E-07 | Vascular Tone |
| Adrenomedullin | +1.6x | 6.19E-09 | Vascular Tone |
| ANGPT1 | −1.7x | 3.06E-04 | Vascular Tone |
| Tissue Factor | −1.4x | 3.36E-06 | Coagulation |
| TM | −1.5x | 1.09E-04 | Coagulation |
| Protein C | −1.7x | 4.01E-05 | Coagulation |
| FGF-23 | +5.2x | 1.04E-12 | Other |
| Pappalysin-1 | +3.1x | 4.02E-05 | Other |
| AMBP | −1.3x | 4.77E-06 | Other |
| hOSCAR | −1.3x | 1.89E-04 | Other |
| KIT | −1.4x | 9.71E-07 | Other |
| CRTAC1 | −1.4x | 1.06E-04 | Other |
| SPARCL1 | −1.4x | 1.35E-04 | Other |
| PRSS 27 | −1.5x | 2.27E-05 | Other |
| IGFBP6 | −1.5x | 4.20E-05 | Other |
Bold signifies concentration higher in cases than controls, Bonferroni corrected P<0.05. Values represent mean fold difference in protein concentration between cases and controls. FGF=fibroblast growth factor, ICAM=intercellular adhesion molecule, PSGL=P-selectin glycoprotein ligand, THPO=thrombopoietin, IL=interleukin, CR=complement receptor, IL1RL2=IL1 receptor ligand 2, TNFR SF=tumor necrosis factor receptor superfamily, AGRP=Agouti-related protein, APOM=apolipoprotein M, GIF=gastric intrinsic factor. CA=carbonic anhydrase, ACE=angiotensin converting enzyme, BNP=B-natriuretic peptide, ANGPT=angiopoietin, TM=thrombomodulin, PRSS=serine protease, IGFBP=insulin-like growth factor binding protein, CRTAC=cartilage acidic protein, SPARCL=Sparc-like protein, hOSCAR=osteoclast-associated immunoglobulin-like receptor. N=29 cases and 25 controls.
We found no difference between the evaluated proteomes of the systemic vein and pulmonary vein samples in SVHD cases (n=19) by PLS-DA (accuracy=0.38, R2=0.29, Q2=−0.98). On single variable testing without correction for multiple comparisons, p<0.05 was observed for 43/184 tested protein markers with 11 reaching intermediate significance by FDR (supplemental table 2). After adjusting for multiple comparisons there were no individual proteins that were highly different by Bonferroni between systemic vein and pulmonary vein samples.
Association Between Pre-Stage 2 Catheterization and Immediate Post-Stage 2 Outcomes
We next evaluated whether clinically available pre-operative variables were associated with the post-operative outcomes of interest. Among subjects who ultimately underwent Stage 2 palliation (n=27), there was no significant relationship between pre-operative pulmonary vascular resistance index, mean pulmonary artery pressure, or ventricular end diastolic pressure and 24h Low Sat%, chest tube duration, total chest tube drainage, or ETT.
Proteomic Changes in the Immediate post-Stage 2 Period
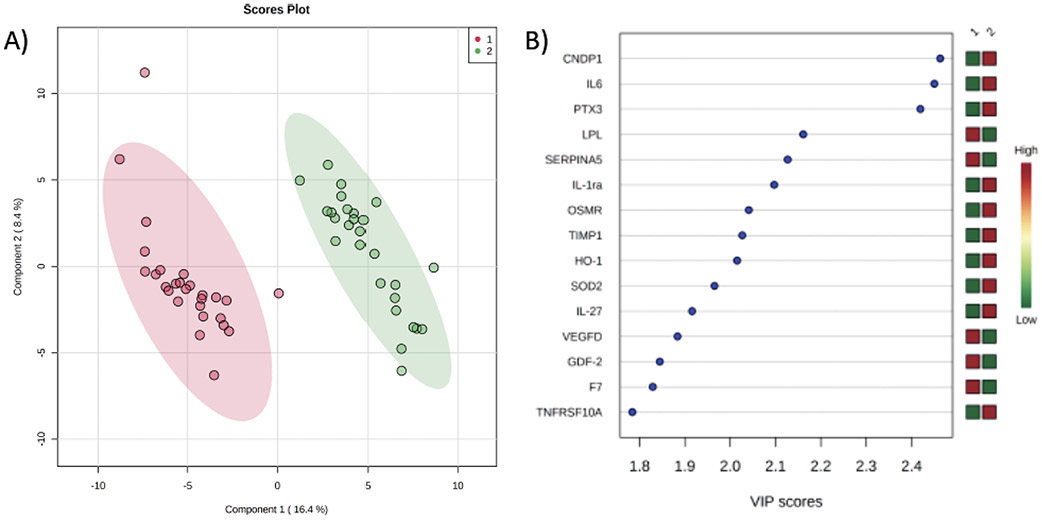
We then compared the evaluated targeted cardiovascular proteome of SVHD cases prior to Stage 2 with the samples obtained 24 hours post-operatively (n=27, figure 2). PLS-DA readily discriminated between pre-operative and post-operative samples (Accuracy=0.98, R2=0.99, Q2=0.92). The 15 markers with greatest effect on the proteomic phenotype between the groups by VIP analysis are shown in figure 2b. On single variable testing without correction for multiple comparisons, p<0.05 was observed for 99/184 tested protein markers with 81 reaching intermediate significance by FDR (supplemental table 3). After Bonferroni correction, 44 tested protein markers showed a highly significant difference (table 3). While many of these proteins are related to inflammation/oxidative stress, several others are best categorized as related to cell metabolism, angiogenesis/vascular tone, or coagulation.
Figure 2:
Comparison of the Proteomic Signature between SVHD Cases before Stage 2 (1) and 24-hour Post-Stage 2 (2). A) Partial least squares-discriminant analysis demonstrates a significant global shift in the proteome between pre-op (red circles) and 24h post-op (green circles). Accuracy=0.98, R2=0.99, Q2=0.92. B) Variable importance projection scores for the 15 most important proteins to distinguish the proteome of SVHD cases and controls. CNDP=B-Ala-His dipeptidase, IL=interleukin, PTX=pentraxin-related protein, LPL=lipoprotein lipase, SERPIN=plasma serine protease inhibitor, OSMR=oncostatin-M-specific-receptor, TIMP=tissue inhibitor of metalloproteinases, HO=heme oxygenase, SOD=superoxide dismutase, VEGFD=vascular endothelial growth factor D, GDF=growth/differentiation factor, F7=factor VII, TNFR SF=tumor necrosis factor receptor superfamily.
Table 3.
Proteins with Different Concentration (corrected p<0.05) Among SVHD Cases Between Pre-Op and Post-Op
| Protein | Fold Change | Raw P Value | Category |
|---|---|---|---|
| IL6 | +47.2x | 2.62E-17 | Inflammation |
| IL-1RA | +3.8x | 1.63E-10 | Inflammation |
| PTX3 | +2.9x | 1.05E-15 | Inflammation |
| LCN2 | +2.9x | 1.15E-05 | Inflammation |
| CEACAM 8 | +2.2x | 1.67E-05 | Inflammation |
| CTSL1 | +2.0x | 1.96E-08 | Inflammation |
| IL27 | +1.9x | 2.04E-12 | Inflammation |
| FCGR2A | +1.8x | 1.39E-04 | Inflammation |
| TNFR SF10A | +1.7x | 2.98E-08 | Inflammation |
| TRAILR2 | +1.5x | 9.81E-06 | Inflammation |
| OSMR | +1.4x | 1.49E-09 | Inflammation |
| IGG Fc R IIb | +1.4x | 4.14E-05 | Inflammation |
| SOD2 | +1.2x | 5.15E-08 | Inflammation |
| DPP4 | −1.3x | 3.42E-05 | Inflammation |
| CD4 | −1.4x | 1.07E-05 | Inflammation |
| FAP | −1.4x | 8.88E-06 | Inflammation |
| TNFR SF13B | −1.4x | 4.21E-06 | Inflammation |
| XCL1 | −1.5x | 6.60E-05 | Inflammation |
| Ficolin2 | −1.6x | 2.75E-05 | Inflammation |
| SERPINA5 | −2.3x | 2.39E-11 | Inflammation |
| CA1 | +3.0x | 2.89E-06 | Cell Metabolism |
| CA3 | +1.7x | 4.65E-06 | Cell Metabolism |
| SAA4 | +1.7x | 6.13E-07 | Cell Metabolism |
| Intrinsic Factor | −1.7x | 2.43E-05 | Cell Metabolism |
| FABP2 | −2.0x | 2.12E-07 | Cell Metabolism |
| LPL | −2.3x | 2.86E-13 | Cell Metabolism |
| FGF21 | +6.5x | 3.16E-07 | Vascular Tone |
| BNP | +4.3x | 1.96E-09 | Vascular Tone |
| TIMP1 | +3.0x | 1.16E-08 | Vascular Tone |
| ADM | +1.8x | 5.13E-07 | Vascular Tone |
| Decorin | +1.3x | 2.46E-06 | Vascular Tone |
| GDF2 | −1.7x | 1.34E-08 | Vascular Tone |
| VEGFD | −2.0x | 2.39E-11 | Vascular Tone |
| TPO | +1.4x | 6.57E-06 | Coagulation |
| Factor VII | −1.7x | 5.90E-08 | Coagulation |
| PAR1 | −2.3x | 6.90E-06 | Coagulation |
| CNDP1 | +5.0x | 1.39E-16 | Other |
| HO | +2.3x | 1.54E-09 | Other |
| FetuinB | −1.3x | 1.59E-04 | Other |
| NCAM1 | −1.5x | 4.86E-07 | Other |
| BOC | −1.5x | 1.30E-05 | Other |
| HAOX1 | −3.0x | 1.38E-04 | Other |
| Pappalysin-1 | −3.3x | 1.23E-04 | Other |
Bold signifies serum concentration higher in post-op than pre-op Bonferroni corrected P<0.05. Values represent mean fold difference in protein concentration between pre-op and post-op. IL=interleukin, PTX=pentraxin-related protein, IL-1RA=IL-1 receptor antagonist, SERPIN=serine protease inhibitor, OSMR=oncostatin-M-specific-receptor, FCGR=Fc region gamma receptor, SOD=superoxide dismutase, TNFR SF=tumor necrosis factor receptor superfamily, CTSL=cathepsin L, LCN=neutrophil gelatinase-associated lipocalin, TRAIL=TNF-related apoptosis-inducing ligand, FAP=prolyl endopeptidase, CEACAM=carcinoembryonic antigen related cell adhesion molecule, DPP=dipeptidyl peptidase, XCL=lymphotactin, LPL=lipoprotein lipase, CA=carbonic anhydrase, SAA=serum amyloid A, FABP=fatty acid binding protein,, TIMP=tissue inhibitor of metalloproteinases, VEGFD=vascular endothelial growth factor D, GDF=growth/differentiation factor, ADM=adrenomedullin, BNP=b-natriuretic factor, FGF=fibroblast growth factor, PAR=proteinase-activated receptor, TPO=thrombopoietin, CNDP=B-Ala-His dipeptidase, HO=heme oxygenase, NCAM=neural cell adhesion molecule, BOC=brother of CDO, HAOX=hydroxyacid oxidase. N = 27.
Association between Individual Proteins and Clinical Outcomes
Dividing the SVHD cohort by the population median for each clinical variable, we evaluated whether the measured pre-operative or post-operative proteome (n=27) was associated with the outcome variables of interest. There was no global difference in the pre-operative systemic venous proteome between patients with more or less post-operative hypoxemia, chest tube drainage, or ETT. No individual systemic vein proteins at the pre-operative time point were statistical predictors of better or worse post-operative clinical outcomes. There were also no global or individual protein differences within the SVHD cohort when divided by patient sex.
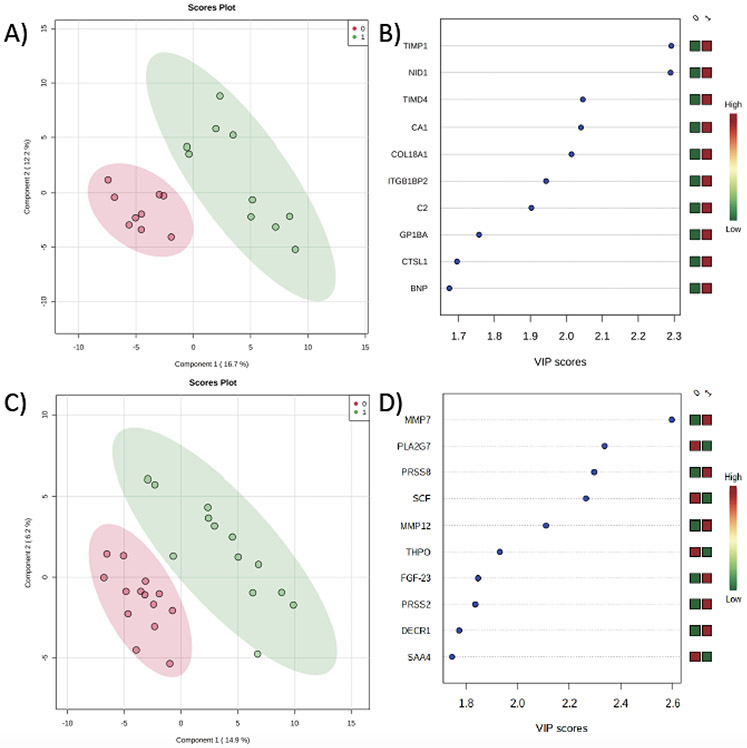
We then evaluated whether the measured pre-operative pulmonary vein proteome was associated with the clinical outcome variables of interest within the SVHD cohort. There was no significant relationship between the pre-operative pulmonary venous proteome and the primary outcome, post-operative hypoxemia burden. The measured pulmonary venous proteome did distinguish between subjects with shorter versus longer post-operative ETT (figure 3a, Accuracy=0.79, R2=0.99, Q2=0.49). The 10 markers with greatest effect on the proteomic phenotype difference between subjects with shorter versus longer ETT are shown in figure 3b. On single variable testing, subjects with longer post-operative ETT (greater than population median 18 hours) had higher levels of the extracellular matrix regulatory proteins tissue inhibitor of metalloproteinase (TIMP) 1 (1.8x fold difference, p=1.6x10−4) and nidogen-1 (NID1, 1.5x fold difference, p=1.7x10−4).
Figure 3:
Comparison of the post-operative proteomic signature between SVHD Cases with better (0) and worse (1) clinical outcomes. A) Partial least squares-discriminant analysis (PLS-DA) demonstrates a distinct pre-operative pulmonary vein (PV) proteome between those with shorter (red circles) and longer (green circles) post-operative intubation time. Accuracy=0.79, R2=0.99, Q2=0.49. B) Variable importance projection scores for the 10 proteins with greatest impact distinguishing between the pre-operative PV proteome of those with shorter v. longer post-op intubation duration. C) PLS-DA demonstrates a distinct post-operative proteome between those with lesser (red circles) and greater (green circles) hypoxemia burden in the first 24 hours after surgery. Accuracy=0.80, R2=0.99, Q2=0.35. B) Variable importance projection scores for the 10 proteins with greatest impact distinguishing between the post-operative proteome of those with lesser v. greater hypoxemia burden. TIMP1=tissue inhibitor of metalloproteinases, NID=nidogen, TIMD=t-cell immunoglobulin and mucin domain protein, CA=carbonic anhydrase, COL=collagen, ITGB1BP2=integrin beta1 binding protein 2, C2=complement 2, GP1BA=Platelet glycoprotein 1b alpha, CTSL=cathepsin, BNP=b-type natriuretic peptide, MMP=matrix metalloproteinase, PLA2G7=platelet activating factor acetyl hydrolase, PRSS8=prostasin, SCF=stem cell factor, THPO=thrombopoietin, FGF=fibroblast growth factor, PRSS2=trypsin 2, DECR1=2,4dienoyl-CoA reductase, SAA=serum amyloid A.
The measured proteome at 24 hours post-operatively distinguished between subjects with more and less post-operative hypoxemia (figure 3c, Accuracy=0.80, R2=0.99, Q2=0.35). Proteins with the greatest effect on the proteomic phenotype between the groups included modulators of the extracellular matrix such as matrix metalloproteinase (MMP) 7 and MMP 12 (figure 3d). On single variable testing, subjects with more post-operative hypoxemia had highly significant changes in levels of MMP 7 (+1.5x fold difference, p=1.97 x 10−5) and platelet activating factor acetylhydrolase (PLA2G7, −1.4x fold difference, p=2.3 x 10−4) compared to those with a lower hypoxemia burden. Reaching intermediate significance by FDR, MMP 12 (p=0.0012, FDR=0.046) and prostasin (p=0.0003, FDR=0.019) levels were also higher in subjects with greater post-operative hypoxemia while stem cell factor (SCF, p=0.0004, FDR=0.019) levels were lower.
Discussion
In this study we used a targeted proteomic approach to evaluate 184 circulating, cardiovascular disease-related proteins in both SVHD patients undergoing Stage 2 palliation and healthy controls. We report (1) downregulation of inflammatory mediators and upregulation of vascular tone modulators in the circulating proteome of inter-stage SVHD infants compared to healthy controls, (2) vast changes in the immediate post-Stage 2 proteome, (3) distinct pre-operative pulmonary vein and post-operative systemic vein proteomes between subjects with more and less favorable immediate post-Stage 2 outcomes, and (4) an association between altered circulating levels of multiple proteins, including extracellular matrix-regulating proteins MMP 7, MMP 12, and TIMP-1, and post-Stage 2 morbidity. This is the first study to evaluate the proteome of infants with SVHD undergoing staged palliation and to identify candidate circulating biomarkers of pulmonary vascular inadequacy in this population.
Inter-Stage SVHD Proteome
The inter-stage period is known to be a high risk time for infants with SVHD.4 Even with optimally balanced systemic and pulmonary blood flow, during inter-stage there is systemic arterial hypoxemia and the single ventricle is volume loaded.2 Further, any perturbation in resistance to pulmonary or systemic flow can lead to significant morbidity either from too much pulmonary perfusion (pulmonary edema, decreased systemic blood flow) or too little (hypoxemia).2
Our finding that the targeted cardiovascular proteome of inter-stage SVHD subjects is readily distinguishable from healthy controls underscores the broad-reaching implications of the stress imposed by inter-stage SVHD physiology. This finding is aligned with prior work demonstrating distinct proteomic patterns in patients with other cardiovascular stressors including children with pulmonary hypertension secondary to CHD, those with ventricular septal defect, and adults with congestive heart failure.14,15,17 Further, the breadth of the biochemical changes seen underscores the importance of systems-based approaches to define complex interactions not well captured by single biomarker strategies.
Focusing on the individual proteins that differ between SVHD infants and healthy controls, three patterns are notable. First, SVHD subjects have decreased concentration of molecules related to circulating platelet and white blood cell adhesion. Specific examples include L-selectin, CD40-ligand, intercellular adhesion molecule 3 (ICAM3), and P-selectin glycoprotein ligand 1 (PSGL-1). Although not previously studied in patients with CHD, prior reports suggest that circulating levels of these molecules may be decreased by treatment with non-steroidal anti-inflammatory agents.18-20 Therefore, these changes may reflect the effect of aspirin therapy that is standard practice for inter-stage SVHD patients at most institutions. Whether these changes truly represent aspirin effect or are markers of altered leukocyte migration in SVHD subjects is an important area for future study.
Second, there is downregulation among SVHD subjects of circulating pro-inflammatory cytokines. Specific examples include interleukin (IL) 18, IL1 receptor ligand 2, and tumor necrosis factor receptor superfamily member 13B (highly significant) along with CXC motif chemokine 1, IL7 receptor, leukocyte immunoglobulin-like receptor subfamily B1, serum amyloid protein A4, and lymphotactin (intermediate significance). These findings align with prior work demonstrating lymphopenia in many SVHD infants before both the stage 2 and stage 3 operations.21 Certain of these individual pro-inflammatory proteins have previously been associated with chronic lung disease; increased IL 18 levels in particular have been implicated in worsening pulmonary fibrosis and pulmonary arterial hypertension.22,23 Decreased circulating pro-inflammatory mediators, and IL 18 in particular, in SVHD cases may reflect an anti-inflammatory protective adaption to inter-stage physiology. These changes could also have implications for maintenance of proper immune function in this population and may be affected by the sub-total thymectomy associated with sternotomy for stage 1 operation in 26 of the 29 SVHD cases.
Third, SVHD subjects have increased circulating levels of mediators of vascular tone. Specific examples include angiotensin converting enzyme 2 (ACE2), adrenomedullin (ADM), and B-type natriuretic peptide (BNP). ACE2 decreases the activity of the vasoconstrictive and pro-remodeling renin-angiotensin-aldosterone (RAA) axis and is protective against both systemic and pulmonary hypertension.24 Higher circulating ACE2 levels are associated with survival among adult subjects with acute respiratory distress syndrome.25 ADM is an endogenous pulmonary vasodilator that also inhibits the RAA axis, decreases pulmonary vascular permeability, and is protective in pulmonary hypertension patients.26,27 Subjects in our SVHD cohort also had increased BNP compared to controls, likely due to the chronic volume load on the single ventricle.28 While frequently measured as a peripheral blood biomarker of heart failure, BNPs direct effect in vivo is to promote diuresis and vasodilation while opposing RAA axis activity in line with the effects of ACE2 and ADM.29 We hypothesize, therefore, that increased circulating ACE2, ADM, and BNP in SVHD subjects represents a counter-regulatory response to elevated neuro-hormonal tone.
Two additional proteins of note showed markedly higher levels in cases than controls. Fibroblast growth factor (FGF) 23, highly involved in phosphate and vitamin D metabolism, is linked to cardiomyocyte hypertrophy, hypoxia response, and pulmonary artery vasoconstriction.30 In the context of prior work showing higher circulating levels associated with adverse hemodynamics in pulmonary hypertension, FGF 23 activation in SVHD patients may reflect a pathologic response.30 Pappalysin-1 is a metalloproteinase produced by several tissues that has been detected in high levels among adult patients with pulmonary pathology including obstructive sleep apnea.31 Not well studied in children, the significance of pappalysin elevation in this population will be an important area for future study.
Proteomic Shifts after Stage 2 Palliation
All SVHD subjects who underwent Stage 2 had their operation performed using cardiopulmonary bypass (CPB). Prior studies investigating the effect of CPB on the proteome of infants have shown significant post-operative changes in proteins involved in systemic inflammation and regulation of coagulation.32-34 Our results mirror these findings with increased concentration (highly significant) of several proteins involved in the inflammatory cascade, including known acute phase reactants IL6, IL27, oncostatin-M-specific receptor (OSMR), and pentraxin-related protein 3 (PTX3) as well as increased levels of IL18, FGF 23, C-C motif chemokines 14 and 18, and IL1 receptor-like2 reaching intermediate significance.
Proteomic analysis also demonstrated changes in multiple proteins involved in regulation of fluid status and vascular tone. Similar to a prior study of the Stage 3 operation for SVHD, BNP concentration rose post-operatively in our population.35 Two key vascular mediators were significantly increased after surgery: ADM and FGF 21. Both are vasodilator molecules with RAA antagonism activity.26,36 A prior study of the RAA axis in subjects with SVHD undergoing stage 2 and 3 operations found that subjects with greater RAA activation had greater risk of post-operative complications, including pleural effusions.37 Activation of ADM and FGF 21 may be a helpful response to balance RAA activity.
Proteomic Phenotype Associated with post-Stage 2 Clinical Outcomes
In this study we report the novel finding that, within the SVHD cohort, proteomic patterns readily distinguish between groups of infants with variable clinical outcomes. Several clinical studies have noted the significant risk SVHD subjects face for post-Stage 2 morbidity.3,6,8,9,38 Our cohort experienced a similar morbidity burden that was not well predicted by conventional markers of post-operative risk such as pulmonary vascular resistance and pulmonary artery pressure. Previous studies have shown an association between an individual’s proteomic fingerprint and disease severity in idiopathic pulmonary fibrosis, chronic heart failure, and end stage renal disease.12,13,39 Our results align with these findings and underscore the potential role of proteomic phenotyping in understanding an individual patient’s clinical risk.
Extracellular Matrix Proteins in Infants Undergoing Stage 2 Palliation
Among the seven proteins associated with unfavorable clinical outcomes in our study, three are modulators of extracellular matrix composition: MMP 7, MMP 12, and TIMP-1. The MMP family of proteins act as proteolytic agents and regulators of the extra-cellular matrix in a variety of tissues.40 Increased circulating concentrations of both MMP 7 and MMP 12 have been implicated in the pro-fibrotic phenotype of chronic remodeling seen in pulmonary arterial hypertension, idiopathic pulmonary fibrosis, and systemic sclerosis with pulmonary involvement patients.41-43 Acutely, increases in circulating levels of multiple MMP family members occur after cardiopulmonary bypass in other populations and are associated with severity of acute lung injury.44-46 Our finding that post-operative MMP concentrations are increased in the subset of SVHD subjects with greater hypoxemia burden may reflect increased post-operative acute lung injury in that sub-cohort. Whether MMP activation persists late after stage 2 palliation and causes chronic extra-cellular matrix remodeling will be an important area for future research.
TIMP-1 is an inhibitor of the MMPs and major regulator of extra-cellular remodeling.47 Although we do not know of any prior study of TIMP-1 measured in human pulmonary veins, increased systemic circulating TIMP-1 is strongly associated with pathologic remodeling in patients with pulmonary hypertension, idiopathic pulmonary fibrosis, and chronic obstructive pulmonary disease.47-49 Our finding that TIMP-1 measured in the pre-operative pulmonary veins but not in the systemic veins was associated with prolonged endotracheal intubation time suggests that certain SVHD patients may have an inter-stage pro-remodeling pulmonary milieu that places those children at higher risk for difficulty separating from the mechanical ventilator.
SCF concentration was decreased post-operatively in subjects with greater hypoxemia burden. In addition to its hematopoietic effects, SCF modulates endothelial permeability and increases endogenous NO production through phosphorylation of NO synthase.50 The role of SCF in the response to stage 2 palliation and cardiopulmonary bypass is an important area for future study.
Limitations
This study has several important limitations. Our center is located approximately 5000 feet above sea level potentially affecting generalizability to centers at lower elevation. As a single center study focusing on a rare disease, the sample size is modest. Because of this and the large number of protein targets selected, the power of subgroup analyses is limited. Variations in clinical practice between subjects including vasoactive medication and blood product exposure may confound our results, including analysis of the post-operative samples in particular. Our data also include a large number of proteins with p values between 0.05 and 0.00027 between the various groups, indicating that the conservative Bonferroni correction is likely underestimating the number of proteins affected both during the inter-stage period and following stage 2 palliation (type 2 error). We therefore additionally report the less-conservative FDR correction as a complement to Bonferroni. Studies using absolute quantification methodologies in larger populations will be important to validate these results. Quantitative mapping of the protein families and their regulators found to be important in this cohort will also be an important area for future work. As this study is focused entirely on the peri-stage 2 period, longitudinal studies evaluating the relationship between change in protein targets over time and clinical outcomes will also be of great interest. Despite similar inclusion criteria, the enrolled control subjects were slightly older and trended toward a greater male predominance than the SVHD cases. Given the biologic plausibility of the proteins identified as different between the groups and the small absolute demographic difference (median 4 months versus 6 months), we believe it is unlikely that these variables are playing a major role in our results. However, future studies focusing on the effect of age and sex on the proteome will be important. This study includes comparison of SVHD patients with healthy controls but no evaluation of non-SVHD CHD patients; comparison of the proteome between subjects with SVHD and those undergoing cardio-pulmonary bypass for repair of biventricular lesions is needed. Given our modest sample size and the multiple factors that can affect the clinical outcomes chosen, the associations noted between proteins of interest and clinical outcomes should be considered hypothesis generating; future studies validating these findings and exploring their pathophysiologic significance will be of great importance.
Conclusions
We report the novel finding that the circulating proteome of inter-stage infants with SVHD differs significantly from healthy controls, showing decreased inflammatory cytokines and increased vascular tone modulators. Twenty-four hours after Stage 2 palliation, SVHD infants demonstrate a significant acute phase reaction as well as increased levels of RAA axis antagonists compared to their pre-operative baseline. Altered post-operative MMP 7, PLA2G7, prostasin, SCF, and MMP 12 and pre-operative TIMP-1 and nidogen-1 are associated with adverse post-operative clinical outcomes.
Supplementary Material
Brief Commentary.
Background
Single ventricle heart disease (SVHD) affects ~1/1000 live births and is fatal without intervention. Clinical monitoring of SVHD infants is limited by poor understanding of underlying pathobiology. Proteomics can identify novel biomarkers in complex disease such as SVHD.
Translational significance
The circulating proteome of inter-stage infants with SVHD differs from healthy controls, showing decreased inflammatory cytokines and increased vascular tone modulators. Twenty-four hours after Stage 2 surgical palliation, subjects demonstrate an acute phase reaction and increased renin-angiotensin-aldosterone axis antagonists. Altered MMP 7, PLA2G7, prostasin, SCF, MMP 12, TIMP-1, and nidogen-1 are associated with adverse post-operative clinical outcomes.
Acknowledgements:
ClinicalTrials.gov Identifier: NCT03404258
Funding:
American Heart Association 18IPA34170070, NIH/NCATS Colorado CTSA Grant Number UL1 TR001082, NIH/NHLBI K23HL123634.
Abbreviations:
- CHD
congenital heart disease
- SVHD
single ventricle heart disease
- CICU
cardiac intensive care unit
- ETT
endotracheal tube time
- PLS-DA
partial least squares – discriminant analysis
- VIP
variable importance in projection
- FDR
false discovery rate
- SpO2
systemic oxygen saturation
- TIMP
tissue inhibitor of metalloproteinase
- NID
nidogen
- MMP
matrix metalloproteinase
- PLA2G7
platelet activating factor acetylhydrolase
- SCF
stem cell factor
- IL
interleukin
- ACE
angiotensin converting enzyme
- ADM
adrenomedullin
- BNP
B-type natriuretic peptide
- RAA
renin-angiotensin-aldosterone
- FGF
fibroblast growth factor
Footnotes
Disclosures: All authors have read the policy on disclosure of potential conflicts of interest and state that they have no conflicts of interest to disclose. All authors have read and are in agreement with the journal’s authorship agreement.
References:
- 1.Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–1900. doi: 10.1016/S0735-1097(02)01886-7 [DOI] [PubMed] [Google Scholar]
- 2.Ohye RG, Schranz D, D’Udekem Y. Current therapy for hypoplastic left heart syndrome and related single ventricle lesions. Circulation. 2016;134(17):1265–1279. doi: 10.1161/CIRCULATIONAHA.116.022816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kogon BE, Plattner C, Leong T, Simsic J, Kirshbom PM, Kanter KR. The bidirectional Glenn operation: A risk factor analysis for morbidity and mortality. J Thorac Cardiovasc Surg. 2008;136(5):1237–1242. doi: 10.1016/j.jtcvs.2008.05.017 [DOI] [PubMed] [Google Scholar]
- 4.Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362(21):1980–1992. doi: 10.1056/NEJMoa0912461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghanayem NS, Allen KR, Tabbutt S, et al. Interstage mortality after the Norwood procedure: Results of the multicenter Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144(4):896–906. doi: 10.1016/j.jtcvs.2012.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.François K, Vandekerckhove K, De Groote K, et al. Current outcomes of the bi-directional cavopulmonary anastomosis in single ventricle patients: Analysis of risk factors for morbidity and mortality, and suitability for Fontan completion. Cardiol Young. 2015;26(2):288–297. doi: 10.1017/S1047951115000153 [DOI] [PubMed] [Google Scholar]
- 7.Brown DW, Gauvreau K, Powell AJ, et al. Cardiac magnetic resonance versus routine cardiac catheterization before bidirectional Glenn anastomosis: Long-term follow-up of a prospective randomized trial. J Thorac Cardiovasc Surg. 2013;146(5):1172–1178. doi: 10.1016/j.jtcvs.2012.12.079 [DOI] [PubMed] [Google Scholar]
- 8.Hansen JH, Uebing A, Furck AK, et al. Risk factors for adverse outcome after superior cavopulmonary anastomosis for hypoplastic left heart syndrome. Eur J Cardio-thoracic Surg. 2011;40(1):43–49. doi: 10.1016/j.ejcts.2011.02.044 [DOI] [PubMed] [Google Scholar]
- 9.Lee TM, Aiyagari R, Hirsch JC, Ohye RG, Bove EL, Devaney EJ. Risk factor analysis for second-stage palliation of single ventricle anatomy. Ann Thorac Surg. 2012;93(2):614–619. doi: 10.1016/j.athoracsur.2011.10.012 [DOI] [PubMed] [Google Scholar]
- 10.Menon SC, McCandless RT, MacK GK, et al. Clinical outcomes and resource use for infants with hypoplastic left heart syndrome during bidirectional glenn: Summary from the joint council for congenital heart disease national pediatric cardiology quality improvement collaborative registry. Pediatr Cardiol. 2013;34(1):143–148. doi: 10.1007/s00246-012-0403-8 [DOI] [PubMed] [Google Scholar]
- 11.Gold L, Ayers D, Bertino J, et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS One. 2010;5(12). doi: 10.1371/journal.pone.0015004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Todd JL, Neely ML, Overton R, et al. Peripheral blood proteomic profiling of idiopathic pulmonary fibrosis biomarkers in the multicentre IPF-PRO Registry. Respir Res. 2019;20(1):1–13. doi: 10.1186/s12931-019-1190-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira JP, Verdonschot J, Collier T, et al. Proteomic Bioprofiles and Mechanistic Pathways of Progression to Heart Failure: The HOMAGE Study. Circ Hear Fail. 2019;12(5):1–12. doi: 10.1161/CIRCHEARTFAILURE.118.005897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Q, Geng Z, Chen T, et al. Comparative proteomic analysis of plasma of children with congenital heart disease. Electrophoresis. 2019;40(14):1848–1854. doi: 10.1002/elps.201900098 [DOI] [PubMed] [Google Scholar]
- 15.Bouwens E, Brankovic M, Mouthaan H, et al. Temporal patterns of 14 blood biomarker candidates of cardiac remodeling in relation to prognosis of patients with chronic heart failure—the Bio-SHiFT study. J Am Heart Assoc. 2019;8(4). doi: 10.1161/JAHA.118.009555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assarsson E, Lundberg M, Holmquist G, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9(4). doi: 10.1371/journal.pone.0095192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Hou HT, Wang J, Liu XC, Yang Q, He GW. Plasma proteomic study in pulmonary arterial hypertension associated with congenital heart diseases. Sci Rep. 2016;6(October):1–11. doi: 10.1038/srep36541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Díaz-gonzález F, Sánchez-madrid F. Europe PMC Funders Group NSAIDs : learning new tricks from old drugs. Eur J Immunol. 2016;45(3):679–686. doi: 10.1002/eji.201445222.NSAIDs [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin HL, Yen HW, Hsieh SL, An LM, Shen KP. Low-dose aspirin ameliorated hyperlipidemia, adhesion molecule, and chemokine production induced by high-fat diet in sprague-Dawley rats. Drug Dev Res. 2014;75(2):97–106. doi: 10.1002/ddr.21159 [DOI] [PubMed] [Google Scholar]
- 20.Valdes V, Nardi M, Elbaum L, Berger J. Reproducibility over Time and Effect of Low-Dose Aspirin on Soluble P-selectin and Soluble CD40 Ligand. J Thromb Thrombolysis. 2015;40(1):83–87. doi: 10.1007/s11239-015-1179-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovacikova L, Krasnanova V, Skrak P, et al. Immune Abnormalities in Patients With Single Ventricle Circulation Precede the Fontan Procedure. World J Pediatr Congenit Heart Surg. 2017;8(6):672–682. doi: 10.1177/2150135117732529 [DOI] [PubMed] [Google Scholar]
- 22.Udjus C, Cero FT, Halvorsen B, et al. Caspase-1 induces smooth muscle cell growth in hypoxia-induced pulmonary hypertension. Am J Physiol - Lung Cell Mol Physiol. 2019;316(6):L999–L1012. doi: 10.1152/ajplung.00322.2018 [DOI] [PubMed] [Google Scholar]
- 23.Kitasato Y, Hoshino T, Okamoto M, et al. Enhanced expression of interleukin-18 and its receptor in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2004;31(6):619–625. doi: 10.1165/rcmb.2003-0306OC [DOI] [PubMed] [Google Scholar]
- 24.Ferreira AJ, Shenoy V, Yamazato Y, et al. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179(11):1048–1054. doi: 10.1164/rccm.200811-1678OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy R, Asante I, Liu S, et al. Circulating angiotensin peptides levels in Acute Respiratory Distress Syndrome correlate with clinical outcomes: A pilot study. PLoS One. 2019;14(3):1–11. doi: 10.1371/journal.pone.0213096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagaya N, Miyatake K, Kyotani S, Nishikimi T, Nakanishi N, Kangawa K. Pulmonary vasodilator response to adrenomedullin in patients with pulmonary hypertension. Hypertens Res. 2003;26(SUPPL.):5–7. doi: 10.1291/hypres.26.S141 [DOI] [PubMed] [Google Scholar]
- 27.Voors AA, Kremer D, Geven C, et al. Adrenomedullin in heart failure: pathophysiology and therapeutic application. Eur J Heart Fail. 2019;21(2):163–171. doi: 10.1002/ejhf.1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eindhoven JA, Van Den Bosch AE, Jansen PR, Boersma E, Roos-Hesselink JW. The Usefulness of brain natriuretic peptide in complex congenital heart disease: A systematic review. J Am Coll Cardiol. 2012;60(21):2140–2149. doi: 10.1016/j.jacc.2012.02.092 [DOI] [PubMed] [Google Scholar]
- 29.Fu S, Ping P, Zhu Q, Ye P, Luo L. Brain natriuretic peptide and its biochemical, analytical, and clinical issues in heart failure: A narrative review. Front Physiol. 2018;9(JUN):1–8. doi: 10.3389/fphys.2018.00692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouzina H, Hesselstrand R, Rådegran G. Higher plasma fibroblast growth factor 23 levels are associated with a higher risk profile in pulmonary arterial hypertension. Pulm Circ. 2019;9(4):1–9. doi: 10.1177/2045894019895446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cengiz A, Konuk S, Tuǧ T. The Relation between Pregnancy-Associated Plasma Protein A and Obstructive Sleep Apnea Syndrome. Can Respir J. 2018;2018. doi: 10.1155/2018/3297810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umstead TM, Lu CJK, Freeman WM, et al. Dual-Platform Proteomics Study of Plasma Biomarkers in Pediatric Patients Undergoing Cardiopulmonary Bypass. Pediatr Res. 2010;67(6):641–649. [DOI] [PubMed] [Google Scholar]
- 33.Umstead TM, Lu CJK, Freeman WM, et al. The kinetics of cardiopulmonary bypass: A dual-platform proteomics study of plasma biomarkers in pediatric patients undergoing cardiopulmonary bypass. Artif Organs. 2012;36(1). doi: 10.1111/j.1525-1594.2011.01412.x [DOI] [PubMed] [Google Scholar]
- 34.Reed CR, McCoy CC, Nag U, et al. Proteomic Analysis of Infants Undergoing Cardiopulmonary Bypass Using Contemporary Ontological Tools. J Surg Res. 2020;246(919):83–92. doi: 10.1016/j.jss.2019.08.019 [DOI] [PubMed] [Google Scholar]
- 35.Kolcz J, Tomkiewicz-Pajak L, Wojcik E, Podolec P, Skalski J. Prognostic significance and correlations of neurohumoral factors in early and late postoperative period after Fontan procedure. Interact Cardiovasc Thorac Surg. 2011;13(1):40–45. doi: 10.1510/icvts.2010.251959 [DOI] [PubMed] [Google Scholar]
- 36.Fisher FM, Maratos-Flier E. Understanding the Physiology of FGF21. Annu Rev Physiol. 2016;78(1):223–241. doi: 10.1146/annurev-physiol-021115-105339 [DOI] [PubMed] [Google Scholar]
- 37.Mainwaring RD, Lamberti JJ, Carter TL, Moore JW, Nelson JC. Renin, Angiotensin II, and the Development of Effusions Following Bidirectional Glenn and Fontan Procedures. J Card Surg. 1995;10(2):111–118. doi: 10.1111/j.1540-8191.1995.tb01228.x [DOI] [PubMed] [Google Scholar]
- 38.Malhotra SP, Ivy DD, Mitchell MB, et al. Performance of cavopulmonary palliation at elevated altitude: midterm outcomes and risk factors for failure. Circulation. 2008;118(14 Suppl):177–182. doi: 10.1161/CIRCULATIONAHA.107.751784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldreich T, Nowak C, Fall T, et al. Circulating proteins as predictors of cardiovascular mortality in end-stage renal disease. J Nephrol. 2019;32(1):111–119. doi: 10.1007/s40620-018-0556-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L. Matrix metalloproteinases: Inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm. 2013;2013. doi: 10.1155/2013/928315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arvidsson M, Ahmed A, Bouzina H, Rådegran G. Matrix metalloproteinase 7 in diagnosis and differentiation of pulmonary arterial hypertension. Pulm Circ. 2019;9(4). doi: 10.1177/2045894019895414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jarman ER, Khambata VS, Ye LY, et al. A translational preclinical model of interstitial pulmonary fibrosis and pulmonary hypertension: Mechanistic pathways driving disease pathophysiology. Physiol Rep. 2014;2(9):1–19. doi: 10.14814/phy2.12133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manetti M, Guiducci S, Romano E, et al. Increased serum levels and tissue expression of matrix metalloproteinase-12 in patients with systemic sclerosis: Correlation with severity of skin and pulmonary fibrosis and vascular damage. Ann Rheum Dis. 2012;71(6):1064–1072. doi: 10.1136/annrheumdis-2011-200837 [DOI] [PubMed] [Google Scholar]
- 44.Guenzinger R, Lahm H, Wottke M, Lange R. Role of metalloproteinases and tissue inhibitors of metalloproteinases during cardiopulmonary bypass in rats. ASAIO J. 2012;58(3):204–211. doi: 10.1097/MAT.0b013e31824709d5 [DOI] [PubMed] [Google Scholar]
- 45.Joffs C, Gunasinghe HR, Multani MM, et al. Cardiopulmonary bypass induces the synthesis and release of matrix metalloproteinases. Ann Thorac Surg. 2001;71(5):1518–1523. doi: 10.1016/S0003-4975(01)02442-0 [DOI] [PubMed] [Google Scholar]
- 46.Eichler W, Bechtel JFM, Schumacher J, Wermelt JA, Klotz KF, Bartels C. A rise of MMP-2 and MMP-9 in bronchoalveolar lavage fluid is associated with acute lung injury after cardiopulmonary bypass in a swine model. Perfusion. 2003;18(2):107–113. doi: 10.1191/0267659103pf662oa [DOI] [PubMed] [Google Scholar]
- 47.Higashimoto Y, Yamagata Y, Iwata T, et al. Increased serum concentrations of tissue inhibitor of metalloproteinase-1 in COPD patients. Eur Respir J. 2005;25(5):885–890. doi: 10.1183/09031936.05.00092804 [DOI] [PubMed] [Google Scholar]
- 48.Safdar Z, Tamez E, Chan W, et al. Circulating Collagen Biomarkers as Indicators of Disease Severity in Pulmonary Arterial Hypertension. JACC Hear Fail. 2014;2(4):412–421. doi: 10.1016/j.jchf.2014.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Todd JL, Vinisko R, Liu Y, et al. Circulating matrix metalloproteinases and tissue metalloproteinase inhibitors in patients with idiopathic pulmonary fibrosis in the multicenter IPF-PRO Registry cohort. BMC Pulm Med. 2020;20(1):1–12. doi: 10.1186/s12890-020-1103-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JY, Choi JS, Song SH, et al. Stem cell factor is a potent endothelial permeability factor. Arterioscler Thromb Vasc Biol. 2014;34(7):1459–1467. doi: 10.1161/ATVBAHA.114.303575 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.