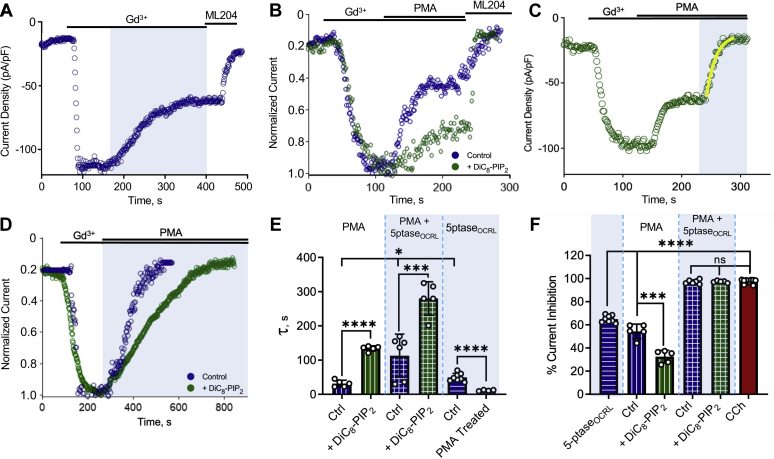
Figure 2.
TRPC5 current inhibition by PKC-mediated phosphorylation and/or PIP2dephosphorylation reveals an underlying decrease in channel–PIP2interactions.A, whole-cell patch clamp recording of HEK293T cells expressing TRPC5–GFP, light-activated CRY2–5’PTASEOCRL, and CIBN–CAAX–GFP (see Experimental procedures); inward current activated by 100 μM GdCl3 with channel current decrease in response to light-activated metabolism of PIP2 and remaining current blocked by 3 μM ML204. B, inhibition observed by PKC activator PMA without/with 200 μM diC8–PIP2 in the pipette. C, HEK-293T cells expressing TRPC5–GFP, CRY2–5’ptase, and CIBN–CAAX–GFP were activated using 100 μM GdCl3; 200 nM PMA was applied to activate PKC enzymes followed by blue-light exposure. D, inhibition observed by simultaneous application of PKC activator PMA and activation of light-activated inositol phosphatase without/with 200 μM diC8–PIP2 in the pipette. E, the bar graph of the mean decay constant ± SD of PMA-mediated inhibition alone (n = 5, 31.44 ± 9.62) and with diC8–PIP2 (n = 5, 133.95 ± 7.766), simultaneous PMA and 5’-ptaseOCRL–mediated inhibition (n = 6, 112.97 ± 62.87) and with diC8–PIP2 (n = 5, 280.33 ± 48.71), and 5’-ptaseOCRL-mediated inhibition alone (n = 8, 52.57 ± 11.59) and after PMA treatment (n = 5, 11.43 ± 1.834). F, the bar graph summary of the mean percentage current inhibition ± SD by 5’-ptaseOCRL (n = 8, 65.18 ± 3.046), PMA-mediated inhibition alone (n = 5, 54.4 ± 7.23), and with diC8–PIP2 (n = 5, 32.47 ± 6.04), simultaneous PMA and 5’-ptaseOCRL-mediated inhibition (n = 6, 97.51 ± 2.397) and with diC8–PIP2 (n = 5, 96.87 ± 0.8152), and when activated using 100 μM CCh (n = 12, 97.61 ± 2.32). Values reported as mean ± SD, p-values established using Students’ t test, comparison with experimental control (#); ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. CCh, carbachol; CRY2, cryptochrome 2; diC8–PIP2, dioctanoyl-glycerol-PIP2; PMA, phorbol 12-myristate-13-acetate; TRPC5, transient receptor potential canonical type 5.