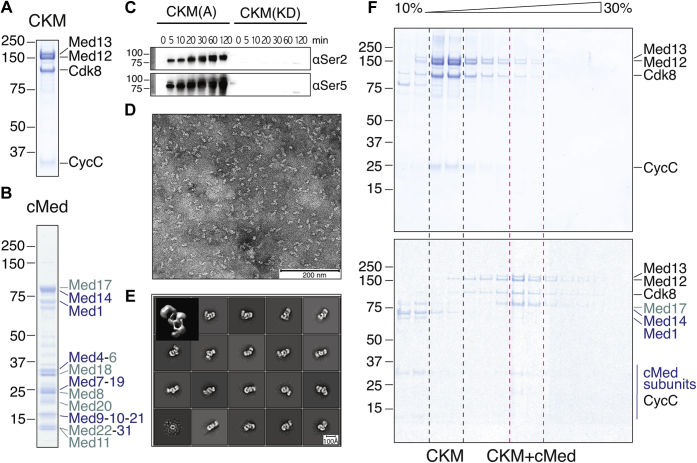
Figure 1.
Purification of recombinant, catalytically active, and structurally homogeneous CKM that binds cMed.A, purified recombinant complete CKM containing its four subunits Med13, Med12, cyclin C, and N-terminally Hexahistidine (His)-maltose binding protein (MBP)–tagged Cdk8 used for affinity capture. B, purified recombinant 16-subunit cMed. C, recombinant CKM is catalytically active as indicated by phosphorylation of one of its known substrates, the pol II C-terminal repeat domain (CTD) at serine 2 and serine 5. Conversely, kinase dead CKM (in which we mutated a critical aspartate in the active site to an alanine D286A) is unable to phosphorylate the pol II CTD under the same experimental conditions. D, purified recombinant CKM according to our newly established method distributes evenly on carbon-foil–coated copper grids stained with uranyl formate and shows particles of the expected size, ranging between 150 and 170 Å along the longest dimension. E, 2D classes and a 3D ab initio reconstruction (top left corner) from negatively stained particle images of approximately 60,000 particles show that purified recombinant CKM is homogeneous and forms a three-lobed overall architecture. F, sucrose density gradient ultracentrifugation of purified CKM (top) and CKM–cMed (bottom) shows a shift in the complex-containing fractions to higher density fractions in the CKM–cMed sample compared with CKM alone, indicating complex formation. CKM, Cdk8 kinase module; cMed, core mediator.