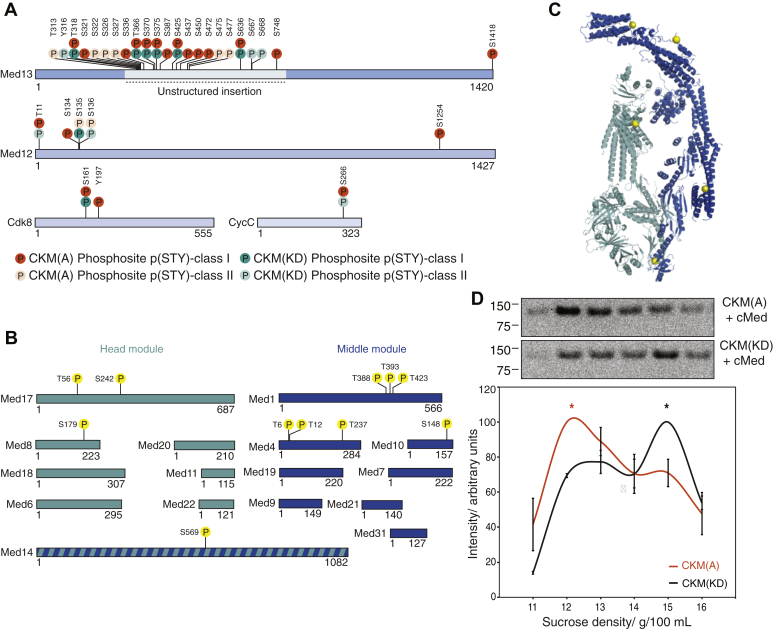
Figure 4.
The CKM phosphorylates itself and cMed on their respective interaction interfaces.A, CKM undergoes extensive intra-CKM phosphorylation by Cdk8 (orange circles) on all its subunits, but particularly on Med13. Phosphorylation sites are shown that have a localization probability greater than or equal to 0.75 (p(STY) class I) or between 0.25 and 0.75 (p(STY) class II) in phosphopeptide enrichment mass spectrometry. B, CKM phosphorylates cMed on head and middle module subunits (yellow circles). Phosphorylation sites are shown that have a localization probability greater than or equal to 0.75. C, phosphorylation sites deposited by the CKM on cMed plotted on the cMed structure (Protein Data Bank ID: 5OQMSaccharomyces cerevisiae cMed–PIC with all other factors hidden) as yellow spheres lie on the CKM–cMed interaction interface. D, analytical sucrose density gradient ultracentrifugation showing that the peak presence of CKM (represented by anti-MBP antibody signal) occurs in lower density fractions when CKM(A) is used under phosphorylation reaction conditions (top), than when CKM(KD) is used under the same conditions (bottom). The mean band intensities from two replicates were quantified and plotted, and the respective peak positions are indicated with an asterisk of the corresponding color. This demonstrates that active phosphorylation by the CKM favors dissociation of the CKM–cMed complex. CKM, Cdk8 kinase module; cMed, core mediator.