Abstract
Background
Healthcare workers (HCWs) are a presumed high-risk population for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. Identifying factors associated with seroprevalence can help establish better practices in healthcare settings. In this study, we evaluate prevalence of SARS-CoV-2 infection among previously undiagnosed HCWs and describe profiling of antibody responses against SARS-CoV-2, including neutralizing antibodies (NAbs).
Methods
We analyzed a cohort of 386 HCWs in a university hospital in Egypt and 725 volunteers not affiliated to any healthcare facility (non-healthcare workers - NHCWs). Participants provided a nasopharyngeal swab and serum samples for SARS-CoV-2 nucleic acid and SARS-CoV-2-specific antibodies, respectively. HCWs who tested positive by either test were sequentially monitored.
Results
At baseline, point prevalence of viral carriage was 11.4% in HCWs (n = 44/386) and 11.9% in NHCWs (86/725). The cumulative prevalence of SARS-CoV-2 infection among HCWs considering all studies was 25.6%, which was statistically lower than in NHCWs (41.0%). Prevalence was greatest among janitorial staff (45.9%) and the most affected departments were gastroenterology (31.1%), and emergency medicine (30.0%). Prior anosmia, fever or headache were associated with higher odds of positivity for SARS-CoV-2 infection. Regarding serial antibody measurements, RT-PCR-positive HCWs displayed IgG detection rates of 29.5%, 70% and 60% at visit 1, visit 2 and visit 3, respectively with slow decline of median IgG antibody titers, whereas, corresponding detection rates for total Ig antibodies were 50%, 90.3%, and 88.9%, respectively with increasing median titers. NAbs measured at each time point were positively correlated with total Ig levels, whereas IgG levels were positively correlated with NAbs at visit 1 and visit 3.
Conclusion
Our results demonstrate lower cumulative prevalence of SARS-CoV-2 infection in HCWs than general population and suggest that asymptomatic HCWs exhibit considerable IgG and total Ig antibodies response as well as NAbs for up to 120 days, with positive correlation in between.
Keywords: COVID-19, SARS-CoV-2, Healthcare workers, Neutralizing antibodies
Introduction
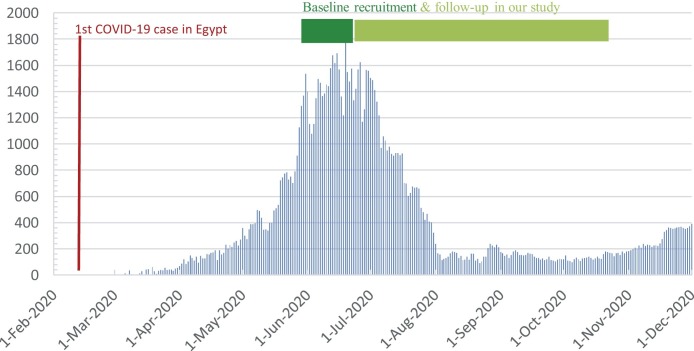
The ongoing pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China in late 2019 [1]. A wide spectrum of coronavirus disease 2019 (COVID-19) has been depicted, ranging from severe cases requiring hospitalization to asymptomatic individuals, who are silent spreaders [2]. Healthcare workers (HCWs) are presumably exposed to a higher risk of acquiring the disease, with previous studies showing infection rates of up to 14% in symptomatic and 7.1% in asymptomatic HCWs [3,4]. A recent meta-analysis found that the proportion of SARS-CoV-2 positive HCWs among all patients with COVID-19 was 10.1%, with variable proportions between countries: China, 4.2%; Italy, 9%; and USA, 17.8% [5]. In Egypt, the first confirmed case was announced on February 14th, 2020 [6], with 2.4% of reported cases in April 2020 being HCWs [7] (Fig. 1 ).
Fig. 1.
Number of officially reported daily new cases according to Ministry of Health and.
Population, Egypt.
Seroprevalence studies and understanding risk factors for SARS CoV-2 infection among HCWs are necessary to assess exposure, safeguard the workforce and maintain healthcare services, particularly in resource-limited health systems [8].
Patients infected with SARS-CoV-2 develop antibodies against virus-specific proteins and antibodies targeting the receptor-binding domains of the spike protein can neutralize the virus [9].
Despite growing knowledge on immunity against SARS-CoV-2, the correlation between quality, quantity and longevity of immune responses and protection against reinfection, remains to be elucidated, so that effective immune-based treatments and vaccines can be developed. [10].
In this study, we evaluated baseline prevalence of SARS-CoV-2 infection via reverse transcription polymerase chain reaction (RT-PCR) and determination of anti-SARS-CoV-2 antibodies within a cohort of previously undiagnosed HCWs and non-HCWs recruited into a study conducted at Kasr Al-Aini University Hospital and we provide description of the dynamic changes of antibodies levels against SARS-CoV-2, including neutralizing antibodies.
Patients and methods
This study was conducted at Kasr Al-Aini University Hospital, Cairo University, a 5600-beds referral hospital complex providing health services in all specialties, with COVID-19 cases served in a dedicated hospital. Eligible HCWs were defined as those who deliver healthcare services to patients in Kasr Al-Aini University Hospital at the time of the study, either directly as physicians or nurses, or indirectly as administrative officers, transporters, or cleaners. Non-healthcare workers (NHCWs) were recruited randomly as volunteers not affiliated to any healthcare facility. Individuals previously tested positive for SARS-CoV-2 via RT-PCR or those self-isolating at home due to symptoms typical of COVID-19 during the last 14 days were excluded from the study.
Ethical committee approval was obtained for the study. After informed consent, participants completed a questionnaire comprising demographics, work location, occupation, medical history, exposure to suspected or confirmed COVID-19 cases, in addition to self-reported prior symptoms compatible with COVID-19.
Molecular detection of SARS-CoV-2 RNA by RT-PCR testing
Nasopharyngeal swabs (NPS) were collected at designated sites and transferred with Universal Transport Media (UTM). SARS-CoV-2 RNA was extracted using QIAGEN extraction kit. The extracted RNA was reverse transcribed into cDNA and amplified in one step using TaqPath™ COVID-19 CE-IVD RT-PCR ComboKit from Thermofisher Scientific, Revision D.0 (Cat.# A48067). Fast Dx Applied Biosystems 7500 real-time thermal cycler was used for amplification. Probes were annealed to three target sequences specific to SARS-CoV-2: ORF1ab, nucleocapsid (N) and spike (S) primers/probes for bacteriophage MS2. Two of the three genes and the MS2 (internal process control) must be positive or the result was considered inconclusive.
SARS-CoV-2 rapid IgM serological test (RST)
Detection of SARS-CoV-2 IgM was done using the lateral flow immunochromatographic assay, COVID-19 IgM/IgG antibody rapid diagnostic test (Artron Laboratories, Burnaby, Canada). The assay has a sensitivity of 93.4% and specificity of 97.7%, as reported by the manufacturer. 10 μL serum was added and incubated for 20–30 s. Subsequently, 2 drops of buffer were added, and the results were detected visually after 15 min. The presence of both the control line and IgM line indicated a positive result for IgM antibody.
Chemiluminescence immunoassay (CLIA)
Plasma samples were prepared from whole blood following centrifugation for 20 min at 2000 g at room temperature. All plasma samples were heat-inactivated for 30 min at 56 °C and stored at −80 °C. IgG antibodies and total Ig antibodies against SARS-CoV-2 were detected using VITROS anti-SARS-CoV-2 IgG test and VITROS anti-SARS-CoV-2 total test, respectively (Ortho Clinical Diagnostics, USA). Both tests are chemiluminescent immunoassays for qualitative detection of either serum IgG or total Ig antibodies (including IgG, IgM and IgA) against spike (S) glycoprotein, with a sensitivity and specificity of 90% and 100% respectively, as reported by the manufacturer. In the first stage antibodies to SARS-CoV-2 present in the sample bind with SARS-CoV-2 spike protein S1 antigen coated on wells. In the second stage horseradish peroxidase (HRP)-labeled murine monoclonal anti-human antibodies are added in the conjugate reagent. The conjugate binds specifically to the antibody portion of the antigen-antibody complex. The bound HRP conjugate is measured by a luminescent reaction, indicating the amount of SARS-CoV-2 antibody present. A cut-off value ≥1 was considered reactive for both antibodies.
Neutralization assays (microneutralization assay)
Serum samples were heat‐inactivated for 30 min at 56 °C. Two‐fold serial dilutions, starting from 1:10 were then mixed with an equal volume of viral solution containing 100 TCID50 of SARS‐CoV‐2. The serum‐virus mixture was incubated for 1 h at 37 °C in a humidified atmosphere with 5% CO2. After incubation, 35 μl of the mixture at each dilution was added in duplicate to a cell plate containing a semi‐confluent vero E6 cell monolayer and incubated for 2 h at the same condition. The inoculum was aspirated and 150 μL medium was added to each well. The plates were incubated for 5 days at 37 °C in a CO2-incubator, before the cultures were inspected under a light microscope for the presence of a cytopathic effect (CPE). Neutralizing antibody titers were expressed as the reciprocal of the last dilution of serum that completely inhibited virus-induced CPE.
Finally, HCWs who tested positive for SARS-CoV-2 infection by either nucleic acid detection test or serological tests were sequentially monitored: 2 months later (visit 2) and another 2 months afterwards (visit 3).
Statistical analysis
Statistical analyses and graphical presentations were performed using GraphPad Prism software version 9.0 (San Diego, USA). Within HCWs, frequencies of positive test results were compared in relation to demographic and clinical characteristics using chi-squared tests. Univariate analysis and multiple logistic regression were performed using positivity as the outcome variable. The comparison between groups was done using Wilcoxon signed-rank test, Mann–Whitney U test, and Krusal–Wallis test to assess changes in antibody titers at baseline and follow-up periods as appropriate. Correlations between obtained different antibody titers were estimated by Spearman rank correlation coefficients. For all analyses, a significance level of 5% was established.
Results
From June 1st to June 14th, 2020, a total of 1111 subjects participated in the study (386 HCWs and 725 NHCWs). The studied cohort was predominantly male (69%), with a mean age of 32.2 years in HCWs and 30.5 years in NHCWs. Among NHCWs, 404 individuals (55.7%) were ≤30 years, 226 (31.2%) aged from 31 to 40 years and 95 (13.1%) were older than 40 years, whereas, among HCWs, 208 (53.9%) were ≤30 years, 139 (36%) aged from 31 to 40 years and 39 (10.1%) were older than 40 years. Co-morbidities were reported in 6.4% of the participants and 62.5% reported contact with suspected or confirmed COVID-19 cases.
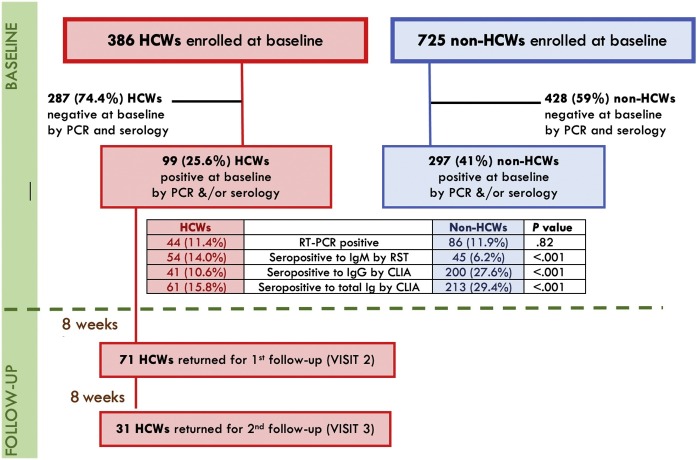
Regarding prevalence of current infection at the time of baseline recruitment, 44/386 HCWs (11.4%) had a positive RT-PCR of SARS-CoV-2, as compared to 86/725 NHCWs (11.9%). In addition, 99 HCWs had either a positive RT-PCR, and/or positive antibodies, raising the cumulative prevalence to 25.6%. A total of 54 (14.0%), 41 (10.6%), and 61 (15.8%) HCWs were seropositive for IgM by RST, IgG by CLIA, and total Ig by CLIA, respectively. This was statistically different from NHCWs in whom, the cumulative prevalence was 41.0% and the seropositivity for IgM, IgG, and total Ig was 45 (6.2%), 200 (27.6%), and 213 (29.4%), respectively (P value < .001) (Fig. 2 ).
Fig. 2.
Flowchart and prevalence of SARS-CoV-2 of the study cohort. Abbreviations: HCWs: healthcare workers; RT-PCR: reverse transcription polymerase chain reaction; RST: rapid serological test; CLIA: chemiluminescent immunoassay; IgG: immunoglobulin G; IgM: immunoglobulin M.
Of the 44 HCWs who had been PCR-positive, 15 (34.1%) went on to have reactive IgM antibody by RST, 13 (29.5%) had reactive IgG and 22 (50%) had positive total IgG by CLIA. Meanwhile, among PCR-negative HCWs, 39 had reactive IgM antibody, 28 had reactive IgG and 39 had positive total IgG. Despite the relatively high sensitivity of RT-PCR, it can yield false-negative results [11]. Therefore, HCWs with positive antibodies and negative RT-PCR were re-tested a week after the initial NPS, all of whom were persistently negative by RT-PCR.
A comparison of characteristics of HCWs based on SARS-CoV-2 status is presented in Table 1 .
Table 1.
Demographic, occupational and clinical characteristics of healthcare workers tested for SARS-CoV-2 at baseline screening.
| Frequency, n (%) |
Univariable analysis |
Multivariable analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| All HCWs | SARS CoV-2 Negative a | SARS CoV-2 Positive b | P-value c | Odds Ratio (95%CI) | P- value | Odds Ratio (95%CI) | P-value | |
| n = 386 | n = 287 | n = 99 | ||||||
| Age group | ||||||||
| ≤30 years | 208 (53.9%) | 159 (55.4%) | 49 (49.5%) | 0.07 | 1 | – | 1 | – |
| 31−40 years | 139 (36.0%) | 95 (33.1%) | 44 (44.4%) | 1.50 (0.93−2.43) | 0.09 | 1.2 (0.65−2.35) | 0.51 | |
| >40 years | 39 (10.1%) | 33 (11.5%) | 6 (6.1%) | 0.59 (0.23−1.49) | 0.27 | 0.26 (0.08−0.84) | 0.03 | |
| Gender | ||||||||
| Female | 206 (53.4%) | 148 (51.6%) | 58 (58.6%) | 0.23 | 1 | – | – | – |
| Male | 180 (46.6%) | 139 (48.4%) | 41 (41.4%) | 1.32 (0.84−2.11) | 0.23 | 1.16 (0.62−2.16) | 0.64 | |
| Close contact with suspected or confirmed COVID-19 | ||||||||
| Yes | 308 (79.8%) | 228 (79.4%) | 80 (80.8%) | 0.77 | 1.09 (0.61−1.94) | 0.77 | – | – |
| Health care role | ||||||||
| Physician | 178 (46.1%) | 149 (51.9%) | 29 (29.3%) | <0.001 | 1 | – | 1 | – |
| Nurse | 141 (36.5%) | 99 (34.5%) | 42 (42.4%) | 2.27 (1.33−3.89) | 0.003 | 1.85 (0.89−3.86) | 0.97 | |
| Administrative employee | 30 (7.8%) | 19 (6.6%) | 11 (11.1%) | 2.97 (1.28−6.91) | 0.01 | 3.09 (1.80−8.82) | 0.04 | |
| Patient transporter/Cleaner | 37 (9.6%) | 20 (7.0%) | 17 (17.2%) | 4.37 (2.04−9.33) | <0.001 | 5.94 (2.08−16.96) | 0.001 | |
| Primary unit | ||||||||
| GI setting | 74 (19.2%) | 51 (17.8%) | 23 (23.2%) | 0.02 | 1 | – | 1 | – |
| ER setting | 203 (52.6%) | 142 (49.5%) | 61 (61.6%) | 0.95 (0.54−1.70) | 0.87 | 0.90 (0.46−1.77) | 0.76 | |
| Pediatrics | 34 (8.8%) | 28 (9.8%) | 6 (6.1%) | 0.48 (0.17−1.30) | 0.15 | 0.40 (0.12−1.32) | 0.13 | |
| Oncology | 34 (8.8%) | 30 (10.5%) | 4 (4.0%) | 0.30 (0.09−0.94) | 0.04 | 0.29 (0.08−1.10) | 0.07 | |
| Others | 41 (10.6%) | 36 (12.4%) | 5 (5.1%) | 0.31 (0.11−0.89) | 0.03 | 0.33 (0.96−1.14) | 0.08 | |
| Co-morbidities d | ||||||||
| Present | 48 (12.4%) | 36 (12.5%) | 12 (12.1%) | 0.91 | 0.96 (0.48−1.93) | 0.91 | 1.01 (0.43−2.40) | 0.98 |
| Vaccine history | ||||||||
| BCG | 353 (91.5%) | 260 (90.6%) | 93 (93.9%) | 0.30 | 1.61 (0.64−4.02) | 0.31 | 2.21 (0.71−6.92) | 0.17 |
| Influenza | 82 (21.2%) | 63 (22.0%) | 19 (19.2%) | 0.56 | 0.84 (0.48−1.50) | 0.56 | 0.83 (0.41−1.68) | 0.61 |
| COVID-19 compatible symptoms within previous months | ||||||||
| Any symptom | 295 (76.4%) | 214 (74.6%) | 81 (81.8%) | 0.14 | 1.54 (0.86−2.73) | 0.14 | – | – |
| Fever | 70 (18.1%) | 43 (15.0%) | 27 (27.3%) | 0.01 | 2.13 (1.23−3.68) | 0.01 | 2.56 (1.17−5.59) | 0.02 |
| Myalgia | 137 (35.5% | 94 (32.8%) | 43 (43.4%) | 0.06 | 1.58 (0.99−2.52) | 0.06 | 0.74 (0.36−1.52) | 0.42 |
| Fatigue | 146 (37.8%) | 107 (37.3%) | 39 (39.4%) | 0.71 | 1.09 (0.68−1.75) | 0.71 | 0.53 (0.25−1.14) | 0.10 |
| Headache | 147 (38.1%) | 102 (35.5%) | 45 (45.5%) | 0.08 | 1.51 (0.95−2.40) | 0.08 | 1.94 (1.02−3.69) | 0.04 |
| Loss of appetite | 36 (9.3%) | 18 (6.3%) | 18 (18.2%) | <0.001 | 3.32 (1.65−6.68) | <0.001 | 2.06 (0.74−5.78) | 0.17 |
| Sore throat | 156 (40.4%) | 110 (38.3%) | 46 (46.5%) | 0.15 | 1.39 (0.88−2.21) | 0.15 | 1.56 (0.83−2.92) | 0.17 |
| Rhinorrhea | 102 (26.4%) | 71 (24.7%) | 31 (31.3%) | 0.20 | 1.39 (0.84−2.30) | 0.20 | 0.94 (0.49−1.82) | 0.86 |
| Anosmia | 39 (10.1%) | 10 (3.5%) | 29 (29.3%) | <0.001 | 11.48 (5.34−24.66) | <0.001 | 14.00 (5.36−36.57) | <0.001 |
| Cough | 111 (28.8%) | 77 (26.8%) | 34 (34.3%) | 0.16 | 1.43 (0.87−2.32) | 0.16 | 1.11 (0.56−2.20) | 0.77 |
| Dyspnoea | 61 (15.8%) | 43 (15.0%) | 18 (18.2%) | 0.45 | 1.26 (0.69−2.30) | 0.45 | 0.49 (0.20−1.17) | 0.11 |
| Chest pain | 49 (12.7%) | 33 (11.5%) | 16 (16.2%) | 0.23 | 1.48 (0.78−2.83) | 0.23 | 0.92 (0.37−2.28) | 0.85 |
| Wheezing | 24 (6.2%) | 15 (5.2%) | 9 (9.1%) | 0.18 | 1.81 (0.77−4.29) | 0.18 | 1.45 (0.45−4.70) | 0.54 |
| Abdominal pain | 65 (16.8%) | 44 (15.3%) | 21 (21.2%) | 0.18 | 1.49 (0.83−2.65) | 0.18 | 0.81 (0.36−1.85) | 0.62 |
| Diarrhea | 97 (25.1%) | 71 (24.7%) | 26 (26.3%) | 0.76 | 1.08 (0.64−1.83) | 0.76 | 0.68 (0.32−1.45) | 0.32 |
Abbreviations: HCW: Health Care Worker; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; COVID-19: Coronavirus Disease; CI: Confidence interval; ER: emergency room; GI: gastrointestinal.
Defined as a negative RT-PCR and undetectable antibodies by RST or CLIA.
Defined as a positive RT-PCR and/or detectable antibodies by RST or CLIA.
Number of positive vs. negative, from chi-squared test.
Co-morbidities include heart and liver disease, diabetes, chronic respiratory and renal disease, and cancers.
The median age of HCWs that tested positive for SARS-CoV-2 was similar to the group that tested negative, (32.6 and 32.0 years, respectively). There was no significant association for testing positive with gender, close contact with COVID-19 cases, co-morbidities, BCG or influenza vaccine history. Staff dealing with patients’ transport and cleaning had the highest frequency of SARS CoV-2 infection (45.9%, 17/37 screened), compared to administrative employees (36.7%, 11/30 screened), nurses (29.8%, 42/141 screened), and physicians (16.3%, 29/178 screened). When SARS-CoV-2 test positivity was mapped to the departments where individuals work, higher rates were found in HCWs working in gastroenterology (31.1%, n = 23/74), or emergency medicine (30.0%, n = 61/203) compared to those working in pediatrics (17.6%, n = 6/34), oncology (11.8%, n = 4/34) or other departments.
In total, 81/99 HCWs with positive tests for SARS-CoV-2 reported prior symptoms of possible COVID-19. The most commonly reported symptoms were sore throat (46.5%), headache (45.5%), myalgia (43.4%), fatigue (39.4%) and cough (34.3%). Fever, loss of appetite and anosmia were statistically associated with SARS-CoV-2 positivity.
In the univariable analysis, the odds of being SARS-CoV-2 positive were higher in HCWs who worked as transporters (OR: 4.37, 95% CI: 2.04−9.33), administrative employees (OR: 2.97, 95% CI: 1.28−6.91) or nurses (OR: 2.27, 95% CI: 1.33−3.89). Symptoms more strongly associated with positivity were anosmia (OR: 11.48, 95% CI: 5.34−24.66), loss of appetite (OR: 3.32, 95% CI: 1.65−6.68) and fever (OR: 2.13, 95% CI: 1.22–3.68). After multivariable analysis, working as a patient transporter (OR, 5.94; 2.08−16.96) or administrative employee (OR, 3.09; 1.80−8.82), prior anosmia (OR, 14.00; 5.36−36.57), prior fever (OR, 2.56; 1.17−5.59) or prior headache (OR, 1.94; 1.02−3.69) were associated with higher odds of SARS-CoV-2 positivity.
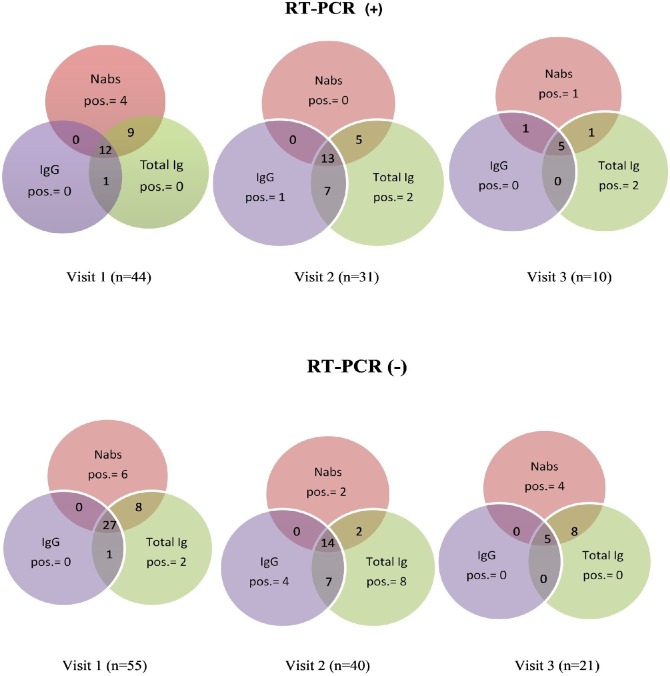
For serial antibody measurements, sequential serum samples were collected from the 99 HCWs tested positive for SARS-CoV-2 infection over a 4-month period between June and October 2020 and were categorized based on baseline RT-PCR results. Notably, the proportion of PCR-positive HCWs who had positive results with three antibodies (IgG, total Ig, and NAbs) increased in each visit from 27.3% (12/44) to 41.9% (13/31) and 50% (5/10) at visit 1, visit 2, and visit 3, respectively indicating that antibodies’ detection rate increased with time since RT-PCR positivity. In contrast, the proportion of PCR-negative HCWs who had positive results with these antibodies in each visit decreased from 49.1% (27/55) to 35% (14/40) and 23.8% (5/21) at visit 1, visit 2, and visit 3, respectively (Fig. 3).
Fig. 3.
Venn diagram displaying positive results with one or more antibodies in each visit over time based on RT-PCR results. The upper row represents RT-PCR positive cases and the lower row represents RT-PCR negative cases.
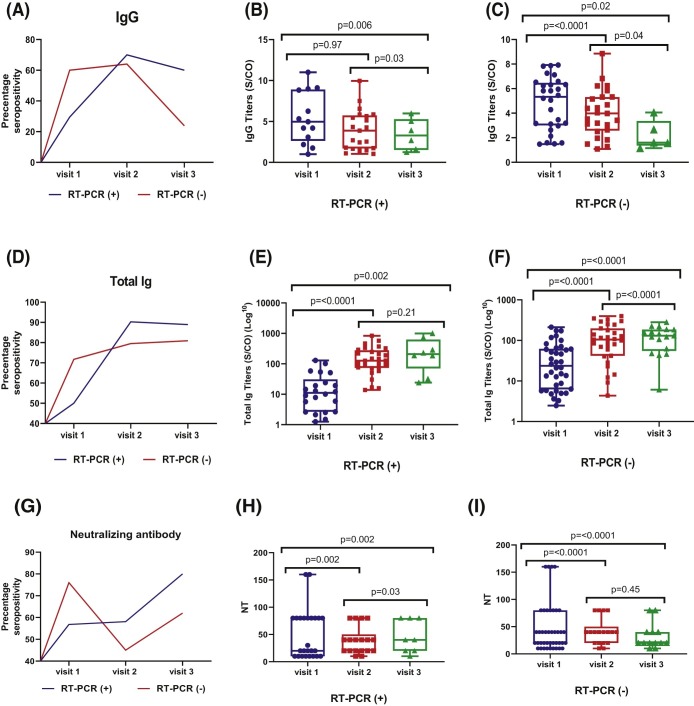
Fig. 4, panel A shows that IgG-positive rates were 29.5% (13/44), 70% (21/30) and 60% (6/10) at visit 1, visit 2 and visit 3, respectively, whereas the corresponding detection rates for total Ig antibody were 50% (22/44), 90.3% (28/31), and 88.9% (8/9) in PCR-positive HCWs. Fig. 4 , panel D shows detection rates of serological assays in PCR-negative HCWs at visit 1, visit 2, and visit 3.
Fig. 4.
Serial percentage of seropositivity and antibodies measurement using serological and neutralization assays based on RT-PCR results at baseline. Horizontal line indicates the median titers of anti-SARS-CoV-2 IgG, total Ig, and neutralizing antibodies. Wilcoxon rank test was used to assess statistically significant differences in antibody levels.
We plotted the changes in median antibody titers at each time point as shown in Fig. 4 (panels B, C for IgG and panels E, F for total Ig). A clear pattern emerged, showing that median titers of IgG antibody displayed a slow decline but titers were still detectable until 120 days (visit 3) in PCR-positive HCWs, whereas median titers of IgG antibody showed a trend of decline until 60 days (visit 2) and were barely detectable thereafter in PCR-negative HCWs. For total Ig, the median titers of total Ig antibody increased continuously and sharply over time up to 120 days regardless RT-PCR results, which were more pronounced in PCR-positive HCWs than those with PCR-negative.
Due to their role in limiting viral spread, we assessed SARS-CoV-2 NAbs generation by asymptomatic HCWs. At baseline visit, 25/44 PCR-positive HCWs (56.8%) and 41/55 of PCR-negative HCWs (74.5%) had developed SARS-CoV-2 NAbs at titers ranging from 1:10 to 1:160. Fig. 4 , panel G shows that median values of NAbs in HCWs with positive PCR results increased and then reached a plateau level up to 120 days while those measured in PCR-negative HCWs remained steady up to 60 days then started to decline slightly at 120 days, as shown in Fig. 4 , panels H, I.
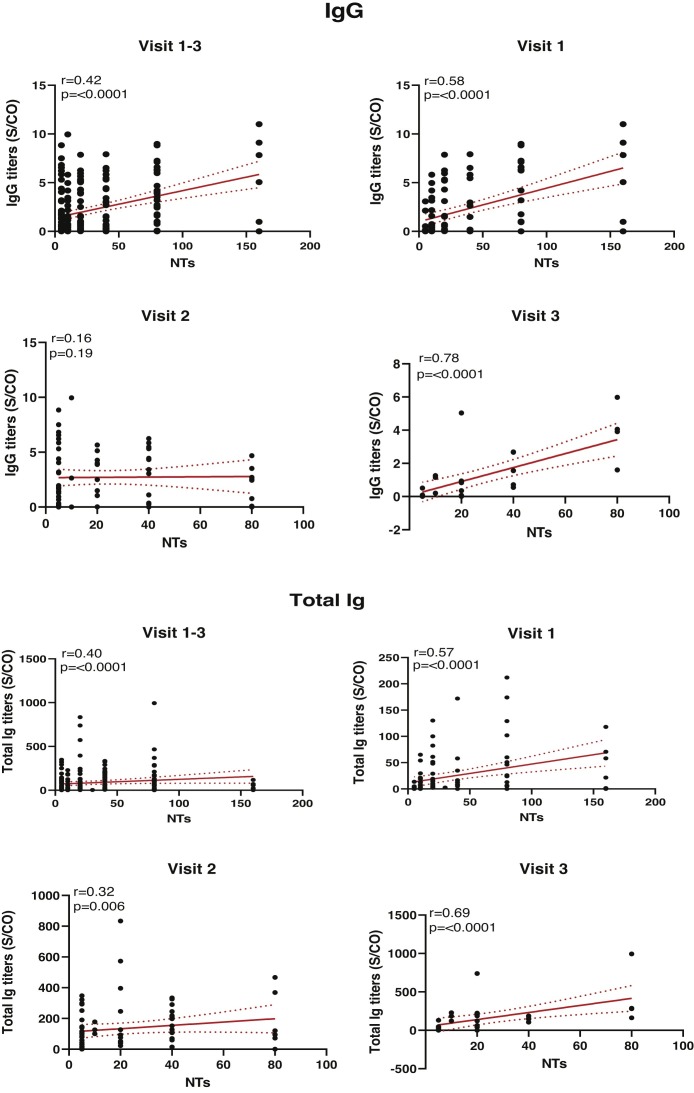
Next, IgG and total Ig antibody levels were evaluated for correlations with NAbs over time. Overall, NAbs values at each time point were positively correlated with total Ig levels, whereas IgG levels were positively correlated with NAbs at visit 1 and visit 3 as shown in Fig. 5 .
Fig. 5.
Correlation analysis between IgG/Total Ig antibodies and neutralizing antibodies at different time point; regression line [solid] and 95% confidence interval [dotted] are given in red. Correlations between different antibody titers were estimated by Spearman’s rank correlation coefficient.
Finally, changes in IgG/total Ig antibodies titers measured using CLIA and the neutralization assay at each visit for 31 of the 99 HCWs who completed 3 visits were individually plotted in Supplementary Fig. 1.
Discussion
In our study, the point prevalence of SARS-CoV-2 nasopharyngeal carriage among asymptomatic HCWs was 11.4%. Although recruitment on a voluntary basis may lead to over-enrollment of HCWs highly concerned about potential infection, our point prevalence keeps with results of a meta-analysis of 97 studies, in which the estimated prevalence of SARS-CoV-2 infection was 11% using RT-PCR [12]. Serological testing by CLIA was performed to evaluate baseline seroprevalence and not as a confirmatory test of infection. In our cohort, 10.6% of HCWs had SARS-CoV-2 IgG antibodies and 15.8% had SARS-CoV-2 total Ig antibodies. Reports from various countries show different seroprevalence patterns among HCWs, with an 8.7% estimated overall seroprevalence of SARS-CoV-2 among HCWs in the meta-analysis by Galanis et al., being higher in studies conducted in North America (12.7%) compared to those in Europe (8.5%), Africa (8.2%) and Asia (4%) [13].
Combining data from current positive RT-PCR and antibody detection, the cumulative prevalence of SARS CoV-2 infection rose to 25.6%, all of whom had not previously been diagnosed with COVID-19, indicating a large percentage of unrecognized infections, possibly because of subclinical infections or underreporting of symptoms. In a university hospital in Spain, 16.9% and 10.8% healthcare workers were SARS-CoV-2 IgG and RT-PCR positive, respectively with a cumulative prevalence 19.9% [14].
Data from many studies support the hypothesis of higher SARS-CoV-2 infection rates in HCWs compared with the general population, suggesting an occupational risk of exposure to SARS-CoV-2 [15,16]. However, this was not the case in our study, where SARS-CoV-2-positivity rates by RT-PCR were found to be similar in HCWs and NHCWs (∼11%) and this was more obvious in seroprevalence data where SARS-CoV-2 seropositivity was significantly higher in non-HCWs in whom the cumulative prevalence of infection was 41%. Given that HCWs are presumably more exposed to the virus, parallel or reversed trends in infection rates in HCWs and the general public may signify community transmission as a greater source of infection especially in presence of peculiar cultural factors in our community. Another contributing factor is that adequate supply of personal protective equipment (PPE), which was the case in our university hospital, is protective and should ease HCWs’ concerns.
Studies examining the occupational role with the highest risk of SARS-CoV-2 infection among HCWs, have shown conflicting results. Some studies have reported high rates of positivity among clinicians [17], nurses [12] or support roles [15], whereas other studies demonstrated no variation in infection rates by job role [18,19]. In our study, we observed higher frequency in janitorial staff and administrative employees, and lower frequency among physicians followed by nurses, who despite more patient-near contact, have greater awareness and training leading to proper PPE use. In order to study the differential influence of work-related exposure within hospital environment, HCWs were classified according to their medical department. Cumulative prevalence of SARS-CoV-2 among HCWs assigned to gastrointestinal and emergency departments was higher (31.1% and 30.0%, respectively) compared to other departments. This seems logical due to more frequent and urgent encounters with patients of unknown SARS-CoV-2 status and generation of infected aerosols during endoscopic procedures [20].
Identifying symptoms associated with SARS-CoV-2 infection, may guide HCWs screening and self-isolation [21]. In our cohort, 10% of HCWs with positive tests for SARS-CoV-2 did not recall any symptoms consistent with COVID-19 in the preceding months. Despite being lower than several studies where 20–50% of infections remain subclinical, this finding supports the inadequacy of relying on symptoms for screening HCWs [22]. Participants were asked to document symptoms over the prior 3–4 months, and although most COVID-19 symptoms are common to many other upper respiratory viral infections, the presence of prior anosmia, fever, or headache were associated with higher odds of positivity. This finding is consistent with several studies that found anosmia to have higher odds of PCR positivity [23]. Although myalgia, fatigue, sore throat and cough have been reported to be associated with SARS-CoV-2 positivity, we did not find significant associations despite their high prevalence in our study population [24].
In the face of SARS-CoV-2 vaccines and viral mutations, it is important to understand the dynamics of immune responses following natural infection. Previous reports observed higher antibody levels earlier in patients with severe/critical infections compared to those with mild/moderate disease [25,26], but less is known about the magnitude and durability of antibody response in asymptomatic SARS-CoV-2 infected individuals [27]. As in other viral infections, SARS-CoV-2 infection has polyclonal elevation of immunoglobulins. Serological tests for SARS-CoV-2 frequently test for IgM antibody isotype to cover early immune responses as well as IgG antibody isotype indicating recovery period or prior infection [28], whereas IgA antibody has been analyzed in few studies [29].
Our results showed that positive rates of SARS-CoV-2 specific IgG /total Ig antibodies in PCR-positive HCWs were higher and maintained over time than in PCR-negative HCWs. Therefore, we hypothesized that those HCWs with negative RT-PCR results had been previously infected by SARS-CoV-2 few weeks prior to study recruitment, when the local spread of SARS-CoV-2 in Egypt was on the rise and the timing of sample collection was later in disease course.
IgG levels declined slowly but remained relatively stable up to 120 days mainly in PCR-positive HCWs. On the other hand, we noted that total Ig levels remained stable and continued to increase up to 120 days regardless PCR results. The immunoglobulin isotype that was elevated to sustain and increase total Ig levels need to be further investigated.
NAbs are considered a key player following natural infection or vaccines [9]. Importantly, we found evidence that asymptomatic individuals with SARS-CoV-2 were capable of generating NAbs at heterogenous titers. The neutralizing activity was maintained at neutralization titers 1:40 up to 120 days in PCR-positive HCWs, in which 80% of them had detectable activity, while those measured in PCR-negative HCWs were maintained up to 60 days and then started to decline at neutralization titers 1:20. This observation has been consistently reported in similar studies as well [30,31].
Another encouraging finding from our study is that neutralization activity was positively correlated to IgG/total Ig antibodies. This might be explained that anti-SARS-CoV-2 IgG/total Ig antibody of our assay could detect neutralizing antibodies directed against the spike (S) protein since IgG/total Ig antibodies target the S1 subunit of the S protein of SARS-CoV-2 which is necessary for viral attachment and entry into host cells [32]. This suggests that levels of IgG/total Ig measured with CLIA can give a clue on the presence of NAbs directed against SARS‐CoV‐2, particularly in vaccinated individuals, without the cost, hazards, time, and expertise needed for neutralizing assays. In the same context, competitive neutralization assays have been designed to replace live-virus-based neutralization test [33].
In conclusion, the cumulative prevalence of SARS-CoV-2 among HCWs was lower than in general population. Our data suggest that asymptomatic PCR-positive HCWs exhibited considerable IgG and total Ig immune responses with detectable levels as well as neutralizing antibodies up to 120 days, with a positive correlation in between. More insights about antibody kinetics in asymptomatic individuals with SARS-COV-2 infection and the possibility of detecting neutralizing antibodies will be of paramount importance for preventing disease transmission and re-infection and addressing the development of effective vaccines.
Authors’ contributions
Sherief Musa: Conceptualization, Writing - review & editing. Shereen Abdel-Alim: Formal analysis, Writing -original draft. Khaled Amer: Investigation. Tarek Elnagdy: Conceptualization, Investigation. Wael A. Hassan: Investigation, Formal analysis. Mohamed A. Ali: Methodology. Yasmine Gaber: Investigation. Hedy A. Badary: Investigation. Omnia Tantawi: Investigation. Reham Abdelmoniem: Investigation. Amr Radwan: Conceptualization. Hanaa Yousof: Formal analysis, Shereen Shawky: Investigation, Resources. Hala Talaat: Supervision. Rabab Fouad: Supervision. Abdel Meguid Kassem: Conceptualization, Funding acquisition, Writing - review & editing. All authors approved the last version.
Trial registration
ClinicalTrials.gov, NCT04424017
Funding
This work was funded by a grant from the Ideation Fund of the Academy of Scientific Research and Technology, Egypt [grant number 7177].
Competing interest
None declared.
Ethical approval
Ethical Committee approvals: ECRRM and Faculty of Medicine, Cairo University
References
- 1.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(March (13)):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y., Tong X., Wang J., Huang W., Yin S., Huang R. High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J Infect. 2020;81(September (3)):420–426. doi: 10.1016/j.jinf.2020.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Treibel T.A., Manisty C., Burton M., McKnight Á, Lambourne J., Augusto J.B. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet. 2020;395(May (10237)):1608–1610. doi: 10.1016/S0140-6736(20)31100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter E., Price D.A., Murphy E., van der Loeff I.S., Baker K.F., Lendrem D. First experience of COVID-19 screening of health-care workers in England. Lancet. 2020;395(May (10234)):e77–e78. doi: 10.1016/S0140-6736(20)30970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahu A.K., Amrithanand V.T., Mathew R., Aggarwal P., Nayer J., Bhoi S. COVID-19 in health care workers — a systematic review and meta-analysis. Am J Emerg Med. 2020;38(September (9)):1727–1731. doi: 10.1016/j.ajem.2020.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO . 2020. Coronavirus disease 2019 (COVID-19) situation report–26.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200215-sitrep-26-covid-19.pdf?sfvrsn=a4cc6787_2 [Google Scholar]
- 7.Papoutsi E., Giannakoulis V.G., Ntella V., Pappa S., Katsaounou P. Global burden of COVID-19 pandemic on healthcare workers. ERJ Open Res. 2020;6(July (2)):00195–02020. doi: 10.1183/23120541.00195-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacob J.T., Baker J.M., Fridkin S.K., Lopman B.A., Steinberg J.P., Christenson R.H. Risk factors associated with SARS-CoV-2 seropositivity among US health care personnel. JAMA Netw Open. 2021;4(March (3)) doi: 10.1001/jamanetworkopen.2021.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel R., Babady E., Theel E.S., Storch G.A., Pinsky B.A., St George K. Report from the American Society for Microbiology COVID-19 International Summit, 23 March 2020: value of diagnostic testing for SARS-CoV-2/COVID-19. mBio. 2020;11(March (2)) doi: 10.1128/mBio.00722-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang A.T., Garcia-Carreras B., Hitchings M.D.T., Yang B., Katzelnick L.C., Rattigan S.M. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11(September (1)):4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West C.P., Montori V.M., Sampathkumar P. COVID-19 testing: the threat of false-negative results. Mayo Clin Proc. 2020;95(June (6)):1127–1129. doi: 10.1016/j.mayocp.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gómez-Ochoa S.A., Franco O.H., Rojas L.Z., Raguindin P.F., Roa-Díaz Z.M., Wyssmann B.M. COVID-19 in health-care workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol. 2021;190(January (1)):161–175. doi: 10.1093/aje/kwaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galanis P., Vraka I., Fragkou D., Bilali A., Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108(February):120–134. doi: 10.1016/j.jhin.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garralda Fernandez J., Molero Vilches I., Bermejo Rodrıguez A., Cano Torres I., Colino Romay E.I., Garcıa Arata I. Impact of SARS CoV-2 pandemic among health care workers in a secondary teaching hospital in Spain. PLoS One. 2021;16(January (1)) doi: 10.1371/journal.pone.0245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eyre D.W., Lumley S.F., O’Donnell D., Campbell M., Sims E., Lawson E. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. Elife. 2020;9(August) doi: 10.7554/eLife.60675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng L., Wang X., Zhou C., Liu Q., Li S., Sun Q. Analysis of the infection status of healthcare workers in Wuhan during the COVID-19 outbreak: a cross-sectional study. Clin Infect Dis. 2020;71(November (16)):2109–2113. doi: 10.1093/cid/ciaa588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lombardi A., Consonni D., Carugno M., Bozzi G., Mangioni D., Muscatello A. Characteristics of 1573 healthcare workers who underwent nasopharyngeal swab testing for SARS-CoV-2 in Milan, Lombardy, Italy. Clin Microbiol Infect. 2020;26(October (10)) doi: 10.1016/j.cmi.2020.06.013. 1413.e9–1413.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vahidy F.S., Bernard D.W., Boom M.L., Drews A.L., Christensen P., Finkelstein J. Prevalence of SARS-CoV-2 infection among asymptomatic health care workers in the Greater Houston, Texas, area. JAMA Network Open. 2020;3(July (7)) doi: 10.1001/jamanetworkopen.2020.16451. [DOI] [PubMed] [Google Scholar]
- 19.Steensels D., Oris E., Coninx L., Nuyens D., Delforge M.L., Vermeersch P. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(July (2)):195–197. doi: 10.1001/jama.2020.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rana S.S. Risk of COVID-19 transmission during gastrointestinal endoscopy. J Dig Endosc. 2020;11(March (1)):27–30. doi: 10.1055/s-0040-1712076. [DOI] [Google Scholar]
- 21.Rudberg A.S., Havervall S., Månberg A., Jernbom Falk A., Aguilera K., Ng H. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11(October (1)):5064. doi: 10.1038/s41467-020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Basteiro A.L., Moncunill G., Tortajada M., Vidal M., Guinovart C., Jiménez A. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11(July (1)):3500. doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roland L.T., Gurrola J.G., 2nd, Loftus P.A., Cheung S.W., Chang J.L. Smell and taste symptom-based predictive model for COVID-19 diagnosis. Int Forum Allergy Rhinol. 2020;10(July (7)):832–838. doi: 10.1002/alr.22602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menni C., Valdes A.M., Freidin M.B., Sudre C.H., Nguyen L.H., Drew D.A. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26(July (7)):1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rijkers G., Murk J.L., Wintermans B., van Looy B., van den Berge M., Veenemans J. Differences in antibody kinetics and functionality between severe and mild severe acute respiratory syndrome coronavirus 2 infections. J Infect Dis. 2020;222(September (8)):1265–1269. doi: 10.1093/infdis/jiaa463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X., Lu S., Li H., Wang Y., Lu Z., Liu Z. Viral and antibody kinetics of COVID-19 patients with different disease severities in acute and convalescent phases: a 6-month follow-up study. Virol Sin. 2020;35(December (6)):820–829. doi: 10.1007/s12250-020-00329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173(September (5)):362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P. Coronavirus infections and immune responses. J Med Virol. 2020;92(April (4)):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pieri M., Ciotti M., Carlozzi N., Frassanito M.L., Meloni A., Cistera A. SARS-CoV-2 infection serology validation of different methods: usefulness of IgA in the early phase of infection. Clin Chim Acta. 2020;511:28–32. doi: 10.1016/j.cca.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(August (8)):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 31.Chen X., Pan Z., Yue S., Yu F., Zhang J., Yang Y. Disease severity dictates SARS-CoV-2-specific neutralizing antibody responses in COVID-19. Signal Transduct Target Ther. 2020;5(September (1)):180. doi: 10.1038/s41392-020-00301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brouwer P.J.M., Caniels T.G., van der Straten K., Snitselaar J.L., Aldon Y., Bangaru S. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020;369(August (6504)):643–650. doi: 10.1126/science.abc5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matusali G., Colavita F., Lapa D., Meschi S., Bordi L., Piselli P. SARS-CoV-2 serum neutralization assay: a traditional tool for a brand-new virus. Viruses. 2021;13:655. doi: 10.3390/v13040655. [DOI] [PMC free article] [PubMed] [Google Scholar]