Abstract
BACKGROUND:
Stroke is responsible for the largest proportion of neurological disorders causing a significant morbidity. Gamma-glutamyl transferase (GGT) enzyme has an active involvement in atherosclerosis through its role in oxidative and inflammatory mechanisms. Recent evidence suggests that serum GGT is related to the risk and prognosis of cerebrovascular diseases.
METHODS:
A total of 200 patients (100 with acute ischemic stroke and 100 without stroke) were recruited from various medical wards and medical intensive care unit. Categorical variables were compared between two groups using Chi-squared test and odds ratio (OR). Independent sample t-test was used to see to compare mean GGT level of stroke patients with control.
RESULTS:
There was no statistical difference with respect to distribution of age groups (χ2 = 1.25, P = 0.741) and gender (χ2 = 2.678, P = 0.1018) between cases and controls. Mean age of stroke patients (mean [M] = 61.7, standard deviation [SD] = 13.8) did not differ from controls (M = 62.2, SD = 13.6), P = 0.80. The odds of suffering from diabetes were significantly higher in stroke patients than cases (OR = 2.25, P = 0.005). Independent sample t-test found a significant difference in serum GGT level between cases (M = 57.47, SD = 11.8) and control (M = 41.72, SD = 7.5), P ≤ 0.0001.
CONCLUSIONS:
Serum GGT was significantly higher in stroke patients than age-sex-matched nonstroke patients. Association remained significant in stroke patients irrespective of age, gender, and other risk factors. Stroke patients with diabetes, hypertension, dyslipidemia, obesity, and smoking had higher level serum GGT level than those without these risk factors. Prospective cohort studies can further explore the potential of GGT as a predictor of acute ischemic stroke.
Keywords: Diabetes, gamma-glutamyl transferase, oxidative, stroke
Introduction
Among all the neurological diseases of adult life, the most frequent and important are cerebrovascular diseases. According to the World Health Organization, stroke is the most common cause of death after heart disease.[1] It is responsible for the largest proportion of neurological disorders which are more often disabling than fatal and results in both physical and mental disability.[2] Community-based studies in India have shown a huge variation in the prevalence of stroke, from 147 to 922/100,000.[3,4] There are several risk factors associated with the stroke of which most important are aging, male sex, smoking, obesity, and dyslipidemia.[5] The identification of risk factors may help clinicians to identify patients at greater risk for stroke and plan early interventions.[6]
There are two types of brain stroke, hemorrhagic, and ischemic. Hemorrhagic type, only 20% of total strokes, is due to rupture of blood vessels. Majority of stroke are ischemic type (80%) and they are due to interruption in the blood supply of the brain due to thrombosis or atherosclerosis.[7,8]
Gamma-glutamyl transferase (GGT) has long been considered an indicator of hepatobiliary dysfunction and alcohol abuse. GGT is present on the cellular membrane and is responsible for intracellular ingress of amino acids and peptides in the form of γ-glutamyl peptides.[9] GGT mediates intracellular uptake of extracellular glutathione, which is produced during normal metabolic processes and thus plays a vital role in protecting the cells from oxidative stress.[10,11] Oxidative stress decreases intracellular glutathione levels and thus induce the formation of GGT enzyme to maintain the normal intracellular glutathione level. However, at times of increased oxidative stress, there is an increased requirement for glutathione and because of the presence of inadequate amounts of glutathione, oxidative stress exerts harmful effects on the cells.
Recent data indicate an active involvement of GGT in the pathogenesis of atherosclerosis through oxidative and inflammatory mechanisms.[12] Oxidative stress predisposes to vascular injury/endothelial dysfunction, leading to atherosclerosis, cardiovascular disease, and stroke.[13] Many studies have found a positive correlation between serum GGT and stroke.[11,14]
Evidence is gradually emerging to suggest that increased serum GGT level is associated with a higher prevalence of silent brain stroke in a neurologically healthy population.[15] It is related to both the risk and prognosis of cerebrovascular diseases.[16]
In population-based studies, after exclusion of alcohol consumption, a positive correlation has been demonstrated between higher GGT levels and advanced age, male gender, increases in body mass index, smoking, sedentary lifestyle, hypertension, tachycardia, hyperglycemia, increased low-density lipoprotein cholesterol, and decreased high-density lipoprotein-cholesterol levels, hypertriglyceridemia, menopause, and oral contraceptive use.[17,18] A recent meta-analysis suggested a high level of GGT increases the risk of stroke independent of alcohol use.[19]
This study was conducted to find out the difference in serum GGT level between Indian patients with acute ischemic stroke and age-sex-matched patients without stroke. The study also examined the association of serum GGT level with various risk factors such as age/gender/hypertension/diabetes/dyslipidemia/smoking/obesity.
Methods
This study was conducted among 100 patients of acute ischemic stroke (cases) in a tertiary care hospital in North India. Another 100 subjects (age and sex matched) who do not have stroke were taken as control. The subjects were selected from the patients attending medical outdoor and admitted in various medical wards and medical intensive care unit from January 2018 to October 2019.
Exclusion criterion were hemorrhagic stroke or extradural hemorrhage or subdural hemorrhage or other space-occupying lesions as identified on the brain imaging study; past history of transient ischemic attacks or cerebrovascular.
Cerebrovascular accident; history of road traffic accidents or other types of traumatic injury; history of use of drugs which might alter GGT levels (phenytoin, barbiturates, fibrates, rifampicin, and oral contraceptives); past history of the coronary vascular event and known cardiac disease and newly onset angina, myocardial infarction, and advanced heart failure; history of chronic liver or renal diseases, diseases of biliary tract or pancreas or gallbladder; carcinoma of prostrate, history of alcohol abuse or dependence.
In control group, subjects attended hospital for various medical problems such as complication of diabetes such as hypoglycemic episodes and ketoacidosis (23), neurological conditions such as neuropathy, myelopathy, and movement disorder (17); headache like migraine and cluster headache (13); worsening of pulmonary conditions such as COPD and asthma (11); gastrointestinal problems such as gastritis and acid peptic disease (8); viral fever like dengue and malaria (8); emergency allergic conditions such as anaphylaxis and angioedema (7); urinary tract infection (6); and hypertensive crisis (4) and others (3). After obtaining informed consent from participants, a semi-structured questionnaire was used to record sociodemographic details and history of various vascular risk factors such as diabetes, hypertension, smoking, alcohol, and dyslipidemia.
Measurement of serum GGT: Randox Gamma GT (Colorimetric Method) (GT3817) system was used for quantitative in vitro determination of L-GGT activity in serum. This product is suitable for use on RX series instruments which includes the RX Daytona and the RX Imola. The colorimetric method is an optimized standard method according to the European Committee for Clinical Laboratory standards.[20]
Statistical analysis
Data collected were analyzed using SPSS software (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp., Released 2012). The significance of the distribution of number was tested using the Chi-square test. Risk factors for stroke were compared between cases and control by calculating odds ratio (OR). The difference in the mean level of serum GGT between different groups was calculated using independent sample t-test. P values lower than 0.05 were considered as statistically significant.
Ethical consideration
The study was approved by the institutional ethics committee (Letter No 2370/Acad-III/MCA/2016 Dated December 18, 2018). Informed consent was obtained from all participants.
Results
The distribution of age group, gender, and risk factors for stroke in study participants is given in Table 1. Males dominated both samples, case and control. There was no statistical difference with respect to the distribution of age groups (χ2 = 1.25, P = 0.741) and gender (χ2 = 2.678, P = 0.1018) between cases and controls. Mean age of stroke cases (mean [M] = 61.7, standard deviation [SD] = 13.8) did not differ from controls (M = 62.2, SD = 13.6), P = 0.80.
Table 1.
Details of participants: Age, gender, and risk factors
| Patients with stroke (n=100) | Patients without stroke (n=100) | Chi-squared test, P | OR (95% CI), P | |
|---|---|---|---|---|
| Age group (years) | ||||
| 21-40 | 8 | 7 | 1.246, 0.742 | |
| 41-60 | 38 | 32 | ||
| 61-80 | 48 | 51 | ||
| >80 | 6 | 10 | ||
| Gender | ||||
| Male | 60 | 71 | 2.677, 0.102 | 0.61 (0.34-1.10), 0.103 |
| Female | 40 | 29 | ||
| Risk factors | ||||
| Diabetes | 62 | 42 | 8.013, 0.005 | 2.25 (1.28-3.97), 0.005 |
| Hypertension | 46 | 46 | 0, 1 | 1.00 (0.57-1.74), 1.000 |
| Dyslipidaemia | 44 | 39 | 0.515, 0.473 | 1.23 (0.70-2.16), 0.473 |
| Obesity | 39 | 35 | 0.343, 0.558 | 1.19 (0.67-2.11), 0.558 |
| Smoking | 62 | 52 | 2.04, 0.153 | 1.51 (0.86-2.64), 0.154 |
OR: Odds ratio, CI: Confidence interval
Prevalence of risk factors was high in both groups, the most common being diabetes and hypertension. However, the odds of suffering from diabetes were significantly higher in stroke patients than cases, OR = 2.25, P = 0.005. Smoking was also more common in cases followed by dyslipidemia and obesity. However, the association for any of them, except diabetes, was found significant as confidence interval was below 1 and P > 0.05. Hypertension was equally common in both groups.
Serum GGT level in different age groups and its association with various risk factors has been shown in Table 2. Distribution of serum GGT and its comparison between stroke patients and control subject is shown in Figures 1 and 2. Independent sample t-test found a significant difference in serum GGT level between cases (M = 57.47, SD = 11.8) and control (M = 41.72, SD = 7.5) conditions; P ≤ 0.0001. The difference remained significant after the separate analysis was done for age groups, gender and risk factors between two groups.
Table 2.
Serum gamma-glutamyl transferase level in cases and control
| Mean serum GGT (SD) | Difference in mean (SD) | t | P | ||||
|---|---|---|---|---|---|---|---|
| Patients with stroke | n=100 | Patients with stroke | n=100 | ||||
| Mean age (SD) | 57.47 (11.8) | 41.72 (7.5) | 15.75 (1.398) | 11.265 | <0.0001 | ||
| Age groups | |||||||
| 21-40 | 52.2 (11.4) | 8 | 39.8 (7.7) | 7 | 12.4 (4.9714) | 2.4284 | 0.0304 |
| 41-60 | 53.9 (8.2) | 38 | 40.1 (7.2) | 32 | 13.8 (1.8411) | 7.4119 | <0.0001 |
| 61-80 | 60.7 (13.4) | 48 | 42.6 (7.7) | 51 | 18.1 (2.2144) | 8.301 | <0.0001 |
| >80 | 60.8 (12.6) | 6 | 43.6 (7.9) | 10 | 17.2 (5.7185) | 3.385 | 0.0044 |
| Gender | |||||||
| Male | 59.5 (11.7) | 60 | 41.4 (7.5) | 71 | 18.1 (1.7532) | 10.695 | <0.0001 |
| Female | 54.3 (11.4) | 40 | 42.5 (7.8) | 29 | 11.800 (2.3123) | 4.8124 | <0.0001 |
| Diabetes | |||||||
| Present | 61.7 (12.5) | 62 | 49.9 (1.18) | 42 | 11.8 (1.5979) | 6.09 | <0.0001 |
| Absent | 50.5 (6.04) | 38 | 35.8 (3.4) | 58 | 14.7 (1.0767) | 15.237 | <0.0001 |
| Hypertension | |||||||
| Present | 66.4 (11.1) | 46 | 42.1 (7.9) | 46 | 24.3 (2.009) | 12.097 | <0.0001 |
| Absent | 49.8 (5.3) | 54 | 41.4 (7.3) | 54 | 8.4 (1.2276) | 6.8425 | <0.0001 |
| Dyslipidaemia | |||||||
| Present | 67.4 (10.5) | 44 | 39.7 (6.9) | 39 | 27.7 (1.9304) | 14.0063 | <0.0001 |
| Absent | 49.6 (4.7) | 56 | 43.4 (7.7) | 61 | 6.2 (1.1689) | 5.2005 | <0.0001 |
| Obesity | |||||||
| Present | 68.5 (10.4) | 39 | 39.2 (6.9) | 35 | 29.300 (2.0331) | 14.1076 | <0.0001 |
| Absent | 50.4 (5.7) | 61 | 43.1 (7.5) | 65 | 7.3 (1.1824) | 6.1213 | <0.0001 |
| Smoking | |||||||
| Present | 62.2 (12.4) | 62 | 40.8 (7.4) | 52 | 21.400 (1.8796) | 10.9165 | <0.0001 |
| Absent | 49.7 (4.5) | 28 | 42.5 (7.6) | 48 | 7.2 (1.388) | 4.5607 | <0.0001 |
t: Independent sample test, SD: Standard deviation, GGT: Gamma-glutamyl transferase
Figure 1.
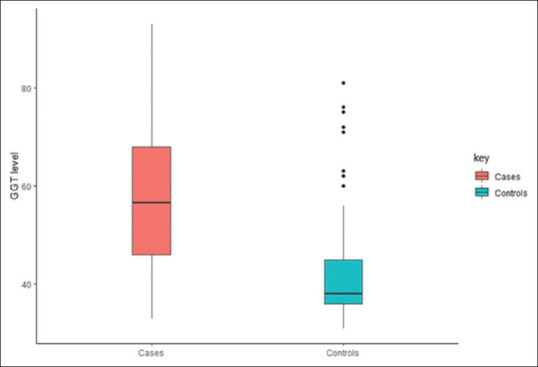
Boxplot – gamma-glutamyl transferase level in stroke patients (cases) and control
Figure 2.
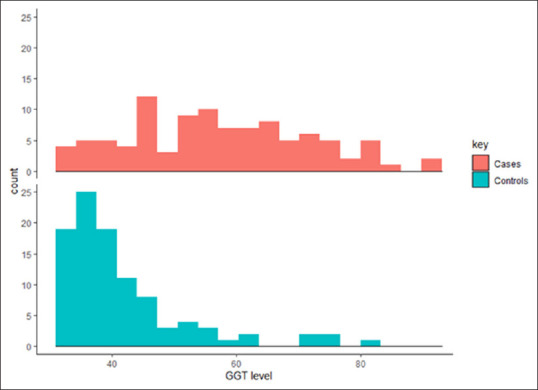
Histogram of gamma-glutamyl transferase level in stroke patient (cases) and control
Table 3 shows the comparison of serum GGT level in cases with respect to gender and the presence of risk factors. Male stroke patients (M = 59.5, SD = 11.7) had significantly higher level than female patients (M = 54.3, SD = 11.4); P = 0.030. Patients with diabetes, hypertension, dyslipidemia, obesity, and smoking had higher serum GGT level than stroke without these risk factors.
Table 3.
Risk factors and serum gamma-glutamyl transferase level in stoke patients
| n=100 | Mean serum GGT (SD) | Difference in mean (SD) | t | P | |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 60 | 59.5 (11.7) | 5.2 (2.3517) | 2.1996 | 0.0302 |
| Female | 40 | 54.3 (11.4) | |||
| Diabetes | |||||
| Present | 62 | 61.7 (12.5) | 11.2 (1.8655) | 5.1592 | <0.0001 |
| Absent | 38 | 50.5 (6.04) | |||
| Hypertension | |||||
| Present | 46 | 66.4 (11.1) | 16.6 (1.7885) | 9.766 | <0.0001 |
| Absent | 54 | 49.8 (5.3) | |||
| Dyslipidaemia | |||||
| Present | 44 | 67.4 (10.5) | 17.8 (1.703) | 11.33 | <0.0001 |
| Absent | 56 | 49.6 (4.7) | |||
| Obesity | |||||
| Present | 39 | 68.5 (10.4) | 18.1 (1.8182) | 11.2272 | <0.0001 |
| Absent | 61 | 50.4 (5.7) | |||
| Smoking | |||||
| Present | 62 | 62.2 (12.4) | 12.5 (1.7358) | 5.9681 | <0.0001 |
| Absent | 38 | 49.7 (4.5) |
t: Independent sample test, SD: Standard deviation, GGT: Gamma-glutamyl transferase
Discussion
Stroke is considered as one of the leading causes of death and disability worldwide.[7,8] Mortality and morbidity associated with stroke can be reduced if patients at risk are identified early, thereby enabling the physician to plan primary prevention strategies promptly. GGT accelerates the process of atherosclerosis through oxidative and inflammatory mechanisms.[10,11,12] As serum GGT is a widely and easily available biochemical test, it was worth exploring and comparing GGT activity in stroke patients with controls.
In this study, stroke patients (cases) were compared with patients without stroke (control). No significant difference was noted with respect to distribution of age groups (χ2 = 1.2465, P = 0.7419) and gender (χ2 = 2.6773, P = 0.1018) between two groups. Ischemic stroke was more prevalent after the age of 40 years. More than half of the stroke patients were males and aged 61–80 years old, as also found in other hospital-based studies.[21,22,23]
This study found that elderly subjects (>60 years) have higher level of serum GGT in both groups. This is a well-known finding seen in many previous studies.[24,25] A study exploring age-related changes in serum GGT activity and ethanol intake found that GGT activities increases with ethanol use but in age-dependent manner.[26] A significant difference in serum GGT level was seen between male and female subjects in the current study. A study by Mijovic et al. in 929 healthy volunteer blood donors also demonstrated that males had higher level of GGT compared to females at all age levels.[27] Previous studies conducted among stroke patients have given mixed findings regarding gender difference in GGT level. While few supports the positive relationship between male gender and GGT activity;[25,28] other do not.[11,24] In a Japanese study conducted by Shimizu et al., serum GGT was positively associated with risk of total stroke for women subjects but not for men.[29]
As expected, the study found that stroke patients have a higher level of serum GGT than control. Although previous studies have shown raised serum GGT in stroke patients, it is important to mention their differences compared to this study. Singh et al. found that mean GGT level in stroke patients was higher (54.95 ± 20.54 IU/L) compared to subjects in control group in their study (32.14 ± 5.07 IU/L).[24] However, control subjects in their study were healthy attendants of patients compared to nonstroke patients in our study and this may explain the lower mean value of serum GGT in their control group compared to ours. Ganesh et al. reported raised serum GGT level in 32 out of 50 subjects with acute stroke.[25] It is important to note here that the above study had patients with both types of stoke and 70% of patients with ischemic stroke had higher serum GGT compared to only 40% with hemorrhagic stroke. Mean GGT level in patients with hemorrahic stroke (28.6 ± 20.5) was much lower than ischemic stroke (51.1 ± 24.0).
In the current study, stroke patients with risk factors had higher serum GGT compared to those without. Previous research also supports positive association of GGT with diabetes, hypertension, smoking, and obesity.[24,30,31]
On comparing serum GGT level in subjects without risk factors, it is seen that stroke patients have significantly higher level compared to control. In case–control study, Korantzopoulos et al. examined 163 elderly patients (>70 years) with first ever acute ischemic/embolic stroke and serum GGT had positive association with stroke independent of established cardiovascular and metabolic risk factors.[30] The “EUROSTROKE PROJECT,” a nested case–control study, revealed that the association between GGT and stroke did not attenuate even after adjusting for variables such as myocardial infarction, total cholesterol, and diabetes mellitus.[28] The elevated levels of GGT in stroke patients could be the result of increased production to decreased levels of intracellular glutathione at times of oxidative stress.
Conclusion
Traditionally, serum GGT is seen as a marker of alcohol use and liver function, this study showed that its level was significantly higher in stroke patients compared to age-sex-matched nonstroke patients. Although patients with established risk factors for stroke had higher level of serum GGT, the association remained significant irrespective of age, gender, and other risk factors such as diabetes, hypertension, dyslipidemia, obesity, and smoking. Therefore, it is worth to further explore the role of measuring GGT level for estimating the risk of stroke, particularly in patient with established risk factors. Prospective cohort studies with large sample size can help establish the potential of GGT as a predictor of acute ischemic stroke.
Limitation of the study
It is hospital-based observation study and therefore causal relationship between serum GGT and stroke cannot established. Moreover, repeat measurement of serum GGT was not done and it may be a source of potential bias. The study might have not included stroke patients who expired or left against medical advice within few hours of reaching emergency and before blood sample collected after reaching emergency ward.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organisation. [[Last accessed on 2021 Jan 18]]. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death .
- 2.Washington, DC, USA: National Academies Press; 2001. Institute of Medicine (US) Committee on Nervous System Disorders in Developing Countries. Neurological, Psychiatric, and Developmental Disorders: Meeting the Challenge in the Developing World. [PubMed] [Google Scholar]
- 3.Prasad K, Vibha D, Meenakshi Cerebrovascular disease in South Asia - Part I: A burning problem. JRSM Cardiovasc Dis. 2012;1:20. doi: 10.1258/cvd.2012.012025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharucha NE, Bharucha EP, Bharucha AE, Bhise AV, Schoenberg BS. Prevalence of stroke in the Parsi community of Bombay. Stroke. 1988;19:60–2. doi: 10.1161/01.str.19.1.60. [DOI] [PubMed] [Google Scholar]
- 5.Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120:472–95. doi: 10.1161/CIRCRESAHA.116.308398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wardlaw J, Brazzelli M, Miranda H, Chappell F, McNamee P, Scotland G, et al. An assessment of the cost-effectiveness of magnetic resonance, including diffusion-weighted imaging, in patients with transient ischaemic attack and minor stroke: A systematic review, meta-analysis and economic evaluation. Health Technol Assess. 2014;18:1. doi: 10.3310/hta18270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marx J, Hockberger R, Walls R. 6th edition. St. Louis: Elsevier Mosby; 2006. Rosen's Emergency Medicine; pp. 1606–19. [Google Scholar]
- 8.Tintinalli JE, Kelen GD, Stapczynski JS Emergency Medicine. 6th edition. New York: McGraw-Hill, Medical Pub; 2004. A Comprehensive Study Guide; pp. 1382–9. [Google Scholar]
- 9.Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, et al. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9:1057–69. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demirkan B, Güray Y, Güray Ü, Turak O, Hajro E, Korkmaz S. The relationship between saphenous coronary bypass graft occlusion and serum gamma-glutamyltransferase activity. Turk Kardiyol Dern Ars. 2010;38:321–6. [PubMed] [Google Scholar]
- 11.Gurbuzer N, Gozke E, Ayhan Basturk Z. Gamma-glutamyl transferase levels in patients with acute ischemic stroke. Cardiovasc Psychiatry Neurol. 2014;2014:170626. doi: 10.1155/2014/170626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ndrepepa G, Colleran R, Kastrati A. Gamma-glutamyl transferase and the risk of atherosclerosis and coronary heart disease. Clin Chim Acta. 2018;476:130–8. doi: 10.1016/j.cca.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1:183–98. [PMC free article] [PubMed] [Google Scholar]
- 14.Koenig G, Seneff S. Gamma-glutamyltransferase: A predictive biomarker of cellular antioxidant inadequacy and disease risk. Dis Markers. 2015;2015:818570. doi: 10.1155/2015/818570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nam KW, Kwon HM, Jeong HY, Park JH, Kim SH, Jeong SM. Serum gamma-glutamyl transferase is associated with silent brain infarcts in a healthy population. Atherosclerosis. 2019;280:45–50. doi: 10.1016/j.atherosclerosis.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Tu WJ, Liu Q, Cao JL, Zhao SJ, Zeng XW, Deng AJ. γ-Glutamyl transferase as a risk factor for all-cause or cardiovascular disease mortality among 5912 ischemic stroke. Stroke. 2017;48:2888–91. doi: 10.1161/STROKEAHA.117.017776. [DOI] [PubMed] [Google Scholar]
- 17.Paolicchi A, Emdin M, Ghliozeni E, Ciancia E, Passino C, Popoff G, et al. Images in cardiovascular medicine.Human atherosclerotic plaques contain gamma-glutamyl transpeptidase enzyme activity. Circulation. 2004;109:1440. doi: 10.1161/01.CIR.0000120558.41356.E6. [DOI] [PubMed] [Google Scholar]
- 18.Tu WJ, Ma GZ, Ni Y, Hu XS, Luo DZ, Zeng XW, et al. Copeptin and NT-proBNP for prediction of all-cause and cardiovascular death in ischemic stroke. Neurology. 2017;88:1899–905. doi: 10.1212/WNL.0000000000003937. [DOI] [PubMed] [Google Scholar]
- 19.Zhang XW, Li M, Hou WS, Li K, Zhou JR, Tang ZY. Association between gamma-glutamyltransferase level and risk of stroke: A systematic review and meta-analysis of prospective studies. J Stroke Cerebrovasc Dis. 2015;24:2816–23. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Leino A, Impivaara O, Irjala K, Mäki J, Peltola O, Järvisalo J. Health-based reference intervals for ALAT, ASAT and GT in serum, measured according to the recommendations of the European Committee for Clinical Laboratory Standards (ECCLS) Scand J Clin Lab Invest. 1995;55:243–50. doi: 10.3109/00365519509089619. [DOI] [PubMed] [Google Scholar]
- 21.Akbar DH, Mushtaq M. Clinical profile of stroke: The experience at King Abdulaziz University Hospital. J Sci Res Med Sci. 2001;3:35–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Wu CY, Wu HM, Lee JD, Weng HH. Stroke risk factors and subtypes in different age groups: A hospital-based study. Neurol India. 2010;58:863–8. doi: 10.4103/0028-3886.73747. [DOI] [PubMed] [Google Scholar]
- 23.Fekadu G, Chelkeba L, Kebede A. Risk factors, clinical presentations and predictors of stroke among adult patients admitted to stroke unit of Jimma university medical center, south west Ethiopia: Prospective observational study. BMC Neurol. 2019;19:187. doi: 10.1186/s12883-019-1409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh LK, Pradhan S, Dash L, Pradhan J, Raul U, Meher RK. Serum gamma- glutamyl transferase level in acute stroke. Int J Res Med Sci. 2019;7:2950–5. [Google Scholar]
- 25.Ganesh RT, Namasivayam K, Vijayakumar G. A study on clinical and prognostic significance of gamma-glutamyl transferase in patients with acute stroke. J Evid Based Med Healthc. 2017;4:3259–62. [Google Scholar]
- 26.Puukka K, Hietala J, Koivisto H, Anttila P, Bloigu R, Niemelä O. Age-related changes on serum ggt activity and the assessment of ethanol intake. Alcohol Alcohol. 2006;41:522–7. doi: 10.1093/alcalc/agl052. [DOI] [PubMed] [Google Scholar]
- 27.Mijovic V, Patapiou H, Machin SJ, McVerry BA, Cleghorn TE. Serum gamma-glutamyl transferase activity in volunteer blood donors. J Clin Pathol. 1977;30:779. doi: 10.1136/jcp.30.8.779-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bots ML, Salonen JT, Elwood PC, Nikitin Y, de Concalves AF, Inzitari D, et al. γ-Glutamyl transferase and risk of stroke: The eurostroke project. J Epidemiol Comm Heal. 2002;56:i25–9. doi: 10.1136/jech.56.suppl_1.i25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu Y, Imano H, Ohira T, Kitamura A, Kiyama M, Okada T, et al. γ-Glutamyltranspeptidase and incident stroke among japanese men and women: The circulatory risk in communities study (CIRCS) Stroke. 2010;41:385–8. doi: 10.1161/STROKEAHA.109.569061. [DOI] [PubMed] [Google Scholar]
- 30.Korantzopoulos P, Tzimas P, Kalantzi K, Kostapanos M, Vemmos K, Goudevenos J, et al. Association between serum gamma-glutamyltransferase and acute ischemic nonembolic stroke in elderly subjects. Arch Med Res. 2009;40:582–9. doi: 10.1016/j.arcmed.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Jousilahti P, Rastenyte D, Tuomilehto J. Serum 15.Gamma-glutamyl transferase, self-reported alcohol drinking, and the risk of stroke. Stroke. 2000;31:1851–5. doi: 10.1161/01.str.31.8.1851. [DOI] [PubMed] [Google Scholar]


