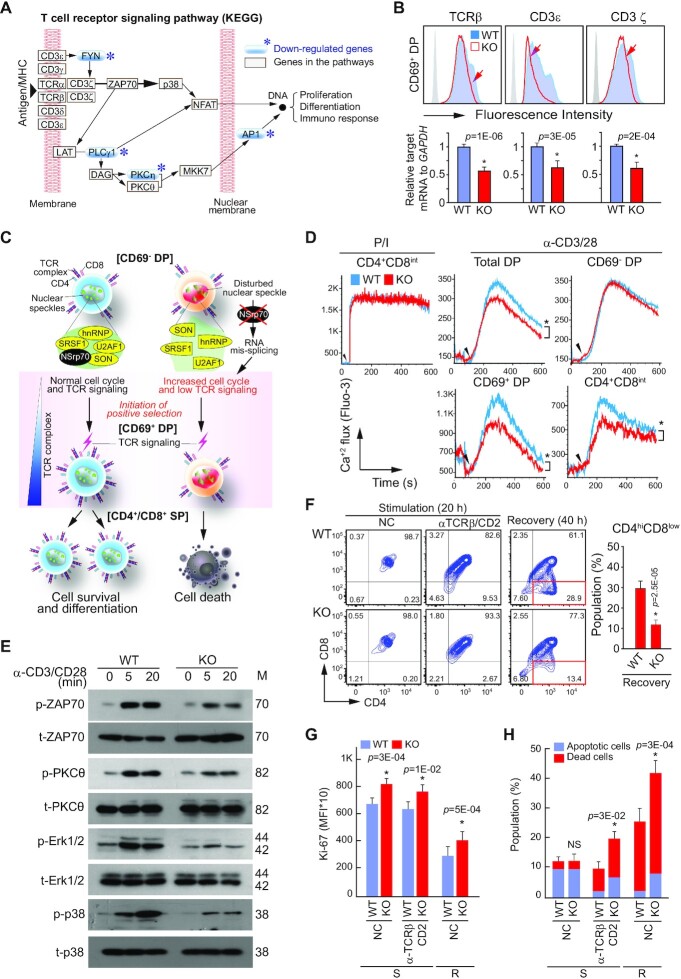
Figure 7.
Deletion of NSrp70 results in defective survival signals following TCR activation in CD69+ DP thymocytes. (A) KEGG T cell receptor signaling pathway. The blue asterisks (*) represent down-regulated genes from the RNA-seq analysis (Figure 5B). (B) Expression of TCRβ, CD3ϵ, and CD3ζ on CD69+ DP thymocytes from Nsrp1f/f (WT) and Nsrp1f/fCD4Cre (KO) mice. (C) A schematic model of gene regulation by NSrp70. NSrp70 sequesters splicing factors in the nuclear speckles. Disintegration of splicing factors by NSrp70 deletion induces abnormal gene regulation during thymocyte development. As one of the results, reduced TCR expression may cause impaired T cell maturation. (D) Calcium flux in DP thymocytes. Cells from (A) were stimulated with PMA and ionomycin (P/I) or anti-CD3/CD28 antibodies, and then calcium fluxes were measured by flow cytometry. (E) Western blot of ZAP70, PKCθ, Erk1/2, and p38 in DP cell lysates stimulated on anti-CD3/28 for 0, 5, and 20 min. β-actin served as the loading control. M, molecular mass (KDa). (F) In vitro thymocyte development assay. CD69– DP thymocytes were stimulated on anti-TCRβ/CD2 antibodies for 20 h (stimulation), or the cells were further incubated for 20 h in medium without stimulation (recovery). (G and H) Cells from (F) were stained for Ki-67 (G) or annexin V and 7ADD (H). Cells were analyzed by flow cytometry (F–H). The bar graphs indicate mean fluorescence intensities (MFI) (G). *, meaningful P-value; NC, non-coated; S, stimulation; R, recovery. The bar graphs indicate average ± standard deviation of apoptotic and dead thymocytes population (H). NS, non-significant P-value. All data shown are representative of three independent experiments.