Figure 5.
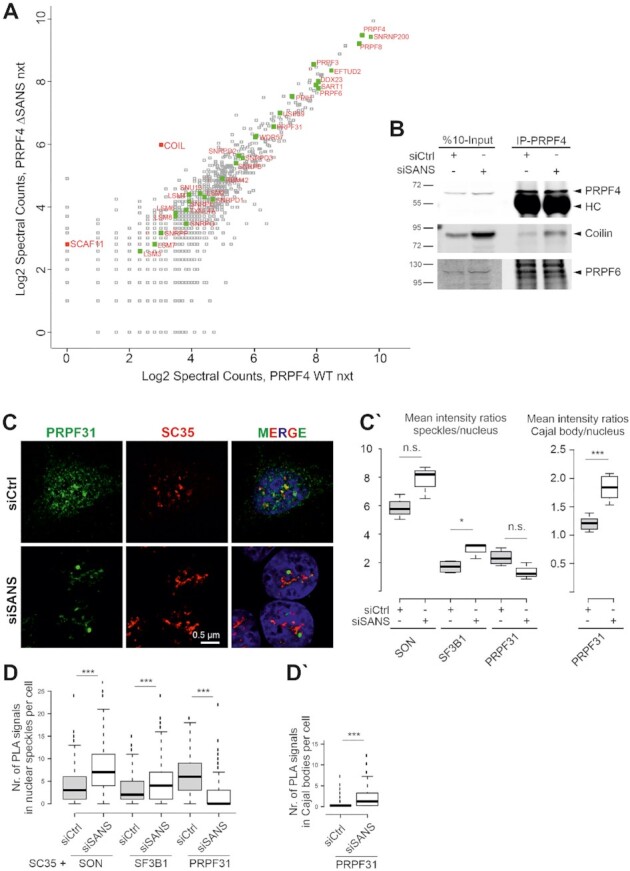
SANS depletion blocks release of tri-snRNP complexes from Cajal bodies and recruitment of mature tri-snRNP complexes to nuclear speckles. (A) Proteomic analysis of precipitated tri-snRNP complexes via FLAG-PRPF4 from nuclear extracts of control cells and SANS-depleted cells. Graph displays peptide spectral counts for each protein in logarithmic scale. Green: tri-snRNPs; red: coilin (COIL) and SC35-interacting protein (SCAF11). There was no significant change in the composition of the tri-snRNP in SANS-depleted nuclear extract, but the tri-snRNP was largely bound to the Cajal body scaffold protein coilin. (B) Western blot analysis of immunoprecipitation of intrinsic PRPF4 from lysates of HEK293T cells demonstrates stronger interactions of the tri-snRNP protein PRPF4 with coilin in SANS-depleted cells. SANS depletion does not affect tri-snRNP complex formation visualized by PRPF4 and PRPF6 interaction. (C) Double immunofluorescence staining reveals reduced PRPF31 staining in SC35 positive nuclear speckles in SANS-depleted cells indicating a defective recruitment of the tri-snRNP complex to nuclear speckles. (C′) Quantification of mean immunofluorescence intensity ratios for PRPF31, SON and SF3B1 observed in nuclear speckles and for PRPF31 in Cajal bodies (CB) versus nucleus. (D) Quantifications of PLA signals for SON-, SF3B1- and PRPF31-SC35 show an increase of SON and SF3B1, but a decrease of PRPF31 in nuclear speckles of SANS-depleted cells. (D′) Quantification of PLA signals for PRPF31-Coilin reveals the accumulation of PRPF31 in Cajal bodies of SANS-depleted cells. ***P values < 0.001, *P values < 0.05.
