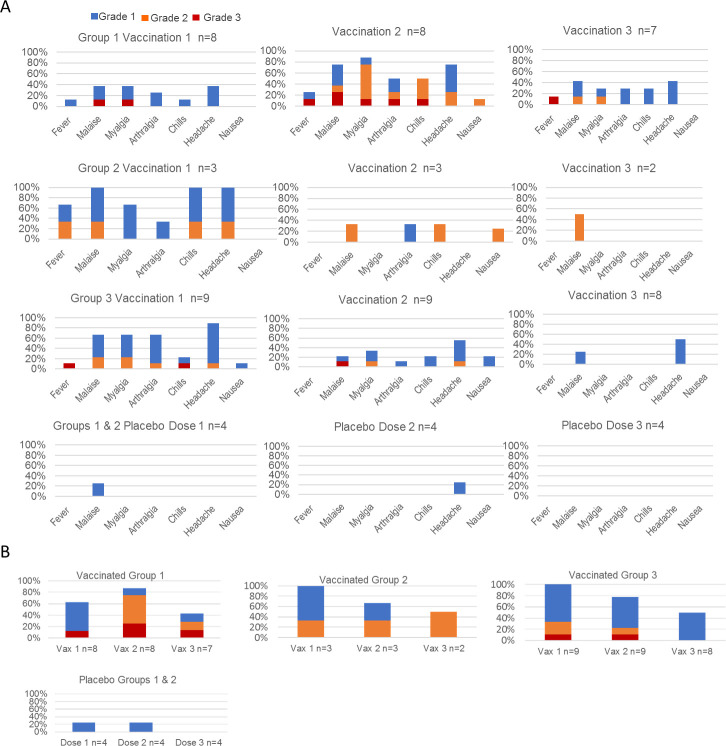
Fig 2. Vaccination phase AEs in vaccinated and placebo control participants.
(A) Specific solicited systemic AEs shown as the proportion of subjects who reported a maximum grade of 3, 2, or 1 in the 7–10 days after each vaccination by group and vaccination status. (B) The proportion of participants in each group and amongst placebo controls who reported at least one Grade 3, Grade 2 (Grade 3 excluded), or Grade 1 (Grades 2–3 excluded) event by dose. Grade 1 events are those that required minimal or no treatment and did not interfere with daily activities. Grade 2 events are those that resulted in a low level of inconvenience or required therapeutic measures and may have interfered with functioning and daily activities. Grade 3 events are those that interrupted the subject’s usual daily activities. Temperature values are noted only if ≥38.0°C (lower limit of graded fever) and are reported as Grade 1 (38.0°C—38.4°C), Grade 2 (38.5°C—38.9°C), or Grade 3 (>38.9°C). Vomiting was a solicited systemic adverse but was not reported by any subject in the 7- to 10- days after a vaccination and so is not represented in the graphs. Blue fill (top), Grade 1; orange fill (middle), Grade 2; red fill (bottom), Grade 3.