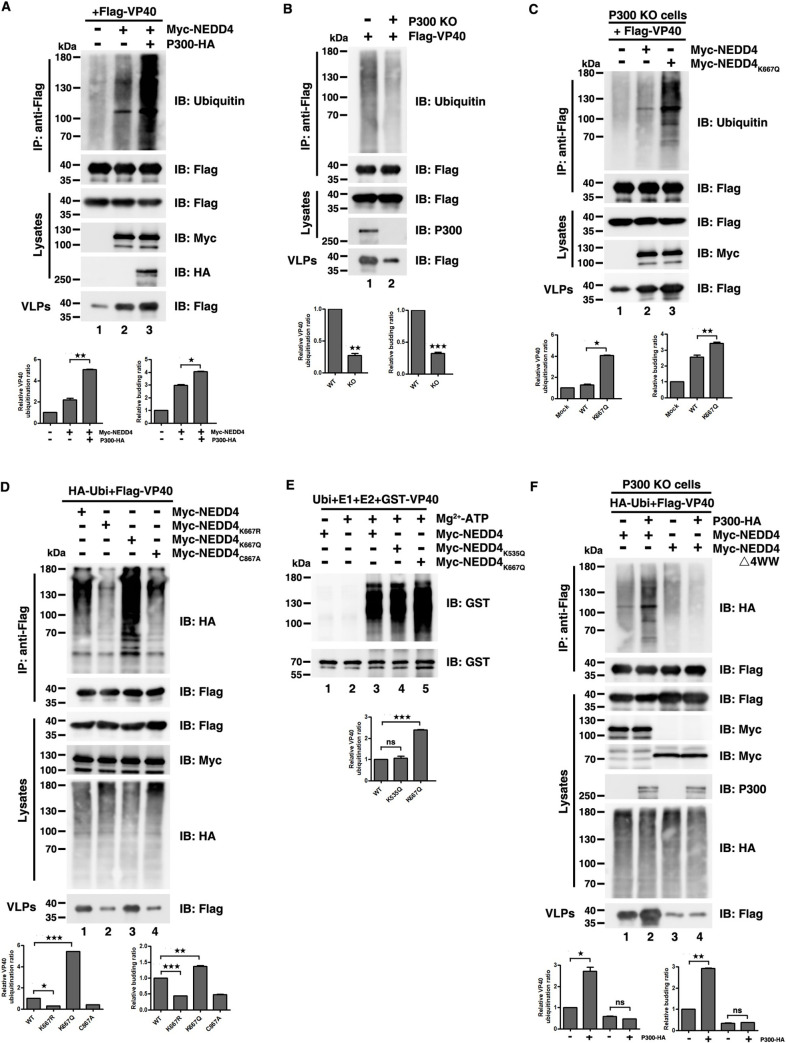
Fig 5. NEDD4 acetylation enhances VP40 ubiquitination.
(A)-(C) Cells were transfected with the indicated plasmid combinations to measure endogenous ubiquitination of VP40 by overexpressing P300-HA in HEK293T cells (A), knocking out endogenous P300 (B), or overexpressing the NEDD4 WT and NEDD4K667Q in P300 KO cells (C). (D) Cells were transfected with the indicated plasmid combinations to measure ubiquitination of VP40 by overexpressing the NEDD4 WT and mutants in HEK293T cells. (E) In vitro ubiquitination assays were used to measure purified GST-VP40 that was incubated with NEDD4 WT, NEDD4K535Q, and NEDD4K667Q, which were immunoprecipitated from HEK293T cells and then analyzed via immunoblotting with an anti-GST antibody to detect VP40 ubiquitination. (F) HEK293T P300 KO cells were transfected with the indicated plasmid combinations to detect the influence of P300 on the ubiquitination of VP40 when the interaction between NEDD4 and VP40 was removed. Error bars, mean ± SD of three experiments. Student’s t test; *p < 0.05; **p < 0.01; ***p < 0.001.