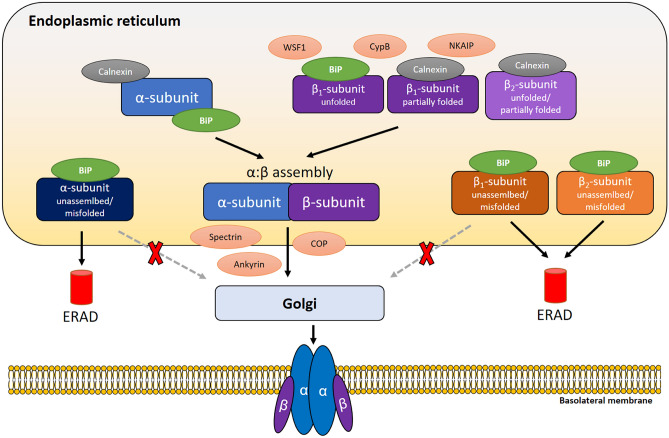
Fig. 1.
Schematic representation of Na,K-ATPase folding in the endoplasmic reticulum. The Na,K-ATPase α- and β-subunits are present in the ER in different physiological or pathophysiological states, including properly folded, unfolded or misfolded subunits of the enzyme and assembled complexes. Calnexin and BiP are ER chaperones that assist the folding of the Na,K-ATPase subunits with isoform specificity and preferential binding properties. Additionally, wolframin (WSF1), cyclophilin B (CypB) and Na,K-ATPase interacting protein (NKAIP) associate with the subunits of the Na,K-ATPase in the ER with divers functions. Only assembled Na,K-ATPase α:β-complexes can exit the ER and transferred to Golgi, whereas unassembled or misfolded subunits are retained with the assistance of BiP and targeted for endoplasmic reticulum-associated degradation (ERAD). Spectrin, ankyrin and coated proteins (COP) assist trafficking of assembled Na,K-ATPase α:β-complexes from the ER to the Golgi apparatus