FIGURE 3.
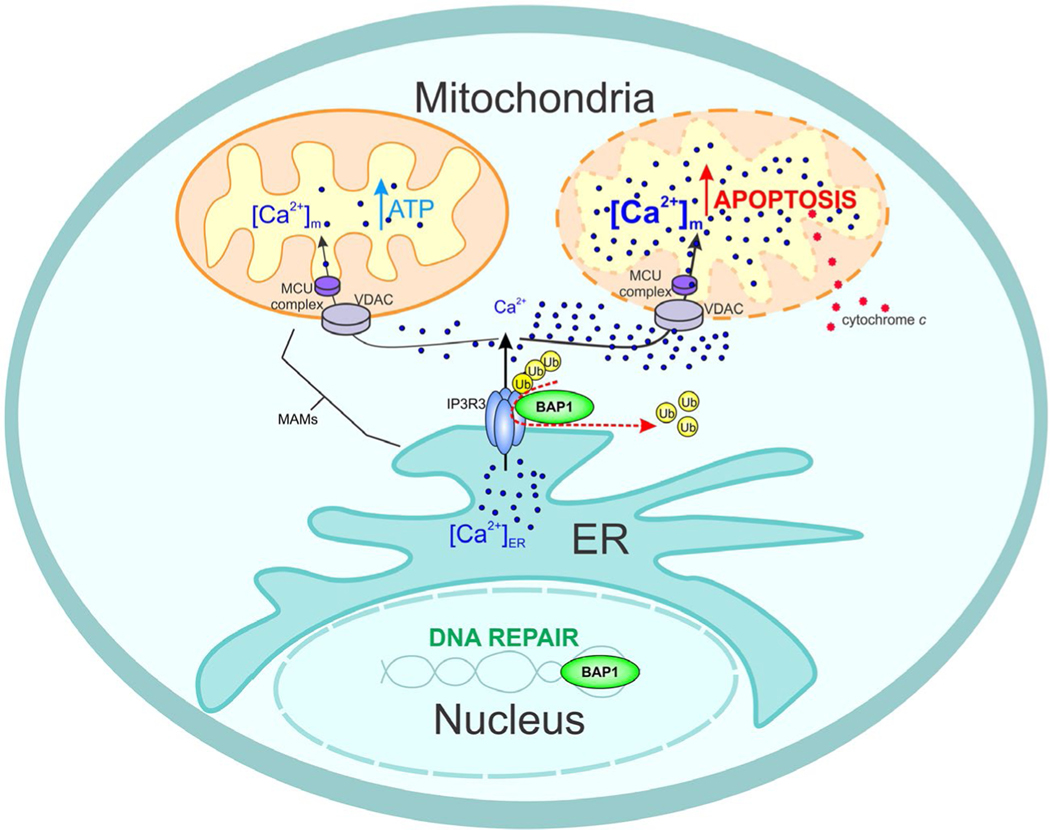
BAP1 Controls Distinct Cellular Activities by Modulating DNA Repair and Ca2+ Intracellular Levels. In the nucleus, BRCA1-associated protein 1 (BAP1) regulates DNA repair. Increased DNA damage is observed in BAP1-mutant cells after exposure to asbestos, ultraviolet light, radiation, and chemotherapy. Similar results are observed in cells in which BAP1 levels are reduced using small-interfering RNA technology. In the cytoplasm, BAP1 deubiquitylates and thus stabilizes the IP3R3 receptor channel that regulates Ca2+ transfer from the endoplasmic reticulum (ER), in which Ca2+ is normally stored in the cell, to the cytoplasm. Ca2+ is released in areas of the ER that are in close contact with the mitochondrial outer membrane: these areas are called MAMs (mitochondrial-associated membranes). Here, Ca2+ flows through the voltage-dependent anion channel (VDAC) channel on the outer mitochondrial membrane and then is actively transported inside the mitochondria by the mitochondrial uniporter channel (MCU) located on the inner mitochondrial membrane. Inside the mitochondria, Ca2+ is required for the normal activity of the Krebs cycle. Reduced Ca2+ concentrations—as in cells carrying BAP1 mutations—impair mitochondrial respiration (Krebs cycle), and the cells switch to aerobic glycolysis (Warburg effect). Normally, when cells sense that DNA damage has occurred and that the damage cannot be repaired, they release higher than normal amounts Ca2+ from the ER through the IP3R3, leading to high mitochondrial Ca2+ concentrations, which, in turn, cause the release of cytochrome c from the mitochondria into the cytosol, in which cytochrome c starts the apoptotic process. Cells with mutated BAP1 cannot release sufficient amounts of Ca2+ to start the apoptotic process. Thus, cells with DNA mutation do not die; instead, they divide and, over time, may become malignant. Ub indicates ubiquitin.