Abstract
Irritable bowel syndrome (IBS) affects about 12% of the global population. Although IBS does not develop into a serious disease or increase mortality, it results in a considerable reduction in the quality of life. The etiology of IBS is not known, but the intestinal microbiota appears to play a pivotal role in its pathophysiology. There is no effective treatment for IBS, and so the applied treatments clinically focus on symptom relief. Fecal microbiota transplantation (FMT), an old Chinese treatment, has been applied to IBS patients in seven randomized controlled trials (RCTs). Positive effects on IBS symptoms in various degrees were obtained in four of these RCTs, while there was no effect in the remaining three. Across the seven RCTs there were marked differences in the selection processes for the donor and treated patients, the transplant dose, the route of administration, and the methods used to measure how the patients responded to FMT. The present frontier discusses these differences and proposes: (1) criteria for selecting an effective donor (superdonor); (2) selection criteria for patients that are suitable for FMT; (3) the optimal FMT dose; and (4) the route of transplant administration. FMT appears to be safe, with only mild, self-limiting side effects of abdominal pain, cramping, tenderness, diarrhea, and constipation. Although it is early to speculate about the mechanisms underlying the effects of FMT, the available data suggest that changes in the intestinal bacteria accompanied by changes in fermentation patterns and fermentation products (specifically short-chain fatty acids) play an important role in improving the IBS symptoms seen after FMT. FMT appears to be a promising treatment for IBS, but further studies are needed before it can be applied in everyday clinical practice.
Keywords: Butyric acid, Enteroendocrine cells, Etiology, Microbiota, Short-chain fatty acids, Superdonor, Therapy
Core Tip: Irritable bowel syndrome (IBS) is a common gastrointestinal disorder. There is no effective treatment for IBS, but fecal microbiota transplantation (FMT) seems promising. Some randomized controlled trials (RCTs) have shown that FMT improved the symptoms and quality of life of IBS patients, but others have found no such effects. The discrepancies between the outcomes of these RCTs are due to the selection processes for the donor and treated patients, the dose of transplant used, and the route of administration. Further studies are needed to optimize the FMT conditions in IBS patients.
INTRODUCTION
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder with a prevalence of 12.1% worldwide[1,2]. Although IBS does not increase mortality, it considerably reduces the quality of life[3]. The etiology of IBS is not known, but several factors appear to be involved in its pathophysiology, including genetics, diet, the intestinal microbiota, enteroendocrine cells, low-grade inflammation, and stress[2,4]. An effective treatment for IBS is lacking, and so the interventions applied to IBS patients clinically are aimed at symptom relief[5].
The human intestine is colonized by more than 10 microorganisms, including archaea, fungi, viruses, and bacteria[6,7], spanning 2172 bacteria species belonging to 12 different bacteria phyla[8]. Bacteria belonging to Firmicutes and Bacteroidetes phyla dominate the bacterial population in the intestine of healthy subjects, and they combine with a few members from the Proteobacteria and Actinobacteria phyla[8,9]. The intestinal bacterial composition varies between individuals due to both genetic and environmental factors[6,10]. These environmental factors include diet, the frequency of antibiotic treatment, the intake of nonantibiotic drugs, geographical location, surgery, smoking, and depression[6,10,11]. A low intestinal bacterial diversity (dysbiosis) is found in several diseases[10,12].
The intestinal bacterial composition in IBS patients deviates from that of healthy subjects[10,13-15]. Compared with healthy subjects, IBS patients have a lower abundance of butyrate-producing bacteria (Erysipelotrichaceae, Ruminococcaceae, Bifidobacterium, Faecalibacterium, and Erysipelotrichaceae spp.) and methanogenic bacteria, and a higher abundance of bacteria belonging to Proteobacteria, Veillonella, and Firmicutes such as Lactobacillus and Ruminococcus spp.[7,16]. In addition, the diversity of intestinal bacteria is lower (dysbiosis) in IBS patients than in healthy subjects[10,13-15,17].
Transplanting the intestinal microbiome (via feces) from a healthy subject with normal bowel function to patients — so-called fecal microbiota transplantation (FMT) — was applied for the first time by the Chinese physician Ge Hong in the fourth century for treating severe diarrhea and malaria[18]. In the present era, FMT represents an effective treatment for Clostridium difficile infection (CDI) and is also a promising invention for other diseases[19-21].
The use of FMT as a treatment for IBS has been investigated in seven randomized controlled trials (RCTs)[11,22-27], which have produced different outcomes. The present frontier aimed at clarifying these differences, highlighting the gaps in our knowledge that need to be filled, and proposing a guideline for successful FMT in IBS patients.
FACTORS THAT AFFECT THE OUTCOME OF FMT
FMT reduced the symptoms and improved the quality of life of the treated IBS patients in four of the seven RCTs[11,22,26,27], but had no effect in the remaining three RCTs[23-25]. These differences in the outcomes of RCTs of FMT in IBS could be explained by differences in the protocols, donor selection, included IBS patients, dose of fecal transplant used, and administration route (Table 1)[11,22-27]. Several suggestions have been made about how to improve the efficacy of FMT in IBS[28,29].
Table 1.
Summary of seven randomized controlled trials of fecal microbiota transplantation for irritable bowel syndrome
|
Study
|
IBS subtypes
|
Number of patients (% female)
|
Intervention (number of patients)
|
Route of administration
|
Primary endpoint
|
Results
|
Microbiota assessment
|
| Johnsen et al[22], 2018 | 53.0% IBS-D, 47.0% IBS-M | 83 (66.3%) | 50-80 g of single FMT (2 donors) (55) vs autologous stool (28) | Colon via a colonoscope | Decrease in IBS severity scoring system of ≥ 75 points at 12 wk and 12 mo | Response rate: 12-wk FMT 65% vs placebo 43% (P < 0.05); 12-mo 56% in FMT vs placebo 36% (P > 0.05) | Unkown |
| Halkjær et al[23], 2018 | 37.3% IBS-M, 33.3% IBS-C, 29.4% IBS-D | 52 (68.6) | 50 g ofdonor stool per dayfor 12 d (4 donors) (26) vs placebo capsules (26) | Oral capsules | Decrease in IBS severity scoring system of ≥ 50 points at 12 wk | Response rate: FMT 36.4% vs placebo 79.2%, P < 0.05 | FMT increased microbiota biodiversity shift to the donor |
| Aroniadis et al[24], 2019 | 100% IBS-D | 24 (37.5) Crossover after 12 wk | 9.5 g of donor stool per day for 3 d (4 donors involved but each participant received from 1 donor) (24) vs placebo capsules (24) | Oral capsules | Decrease in IBS severity scoring system of ≥ 50 points at 12 wk | FMT-first 50% vs placebo-first 61%, (P = 0.46) | Prevotella abundance did not change after FMT; Similar diversity between responders and non-responders |
| Holster et al[25], 2019 | 56% IBS-D, 25.0% IBS-C, 19% IBS-M with a low amount of butyrate producing bacteria in their fecal samples | 17 (50.0%) | 30 g of single FMT (1 donor) (8) vs autologous stool (8) | Colon via a colonoscope | Decrease in gastrointestinal symptom rating scale-IBS of ≥ 30% | Symptom and QoL improved from baseline only in FMT arm (P < 0.05) but not different between arms | Trend of fecal and mucosal microbiota shift to the donor. No significant change in diversity and butyrate-producing bacteria |
| Lahtinen et al[26], 2020 | 51% IBS-D, 28.6% IBS-U. 14% IBS-M, 6% in remission | 49 (59.2%) | 30 g of single FMT (1 donor) (23) vs 30 g of autologous stool (26) | Colon via a colonoscope | decline in the IBS-SSS score of ≥ 50 points throughout 52 wk | Primary endpoint was not achieved in both arms. Only transient improved in FMT arm vs baseline at week 12 | Significant shift in the microbiota profile and richness increased in FMT group |
| El-Salhy et al[11], 2020 | 38.4% IBS-D, 37.8% IBS-C, 23.8% IBS-M | 164 (81%) | single 30 g FMT (54) vs 60 g (55) vs autologous stool (55) | Distal duodenum via a gastroscope | total IBS-SSS score decreased by ≥ 50 points at 3 mo | Response rate: 30 g FMT 76.9%, 60 g FMT 89.1%, control 23.6% P < 0.05; response independent of gender, IBS subtype | At 1 mo: Dysbiosis index/prevalence were not different at baseline vs post FMT (P > 0.05), Responder had higher signals forEubacterium biforme, Lactobacillus spp. and Alistipes spp. After FMT, and lower signals for Bacteroides spp |
| Holvoet et al[27], 2021 | 100% IBS-D or IBS-M with predominant bloating | 62 (64.7) | Single FMT (2 male donors, each participant received from 1 donor) (43) vs autologous stool (19) | Small intestine via a nasojejunal tube | Self-reported adequate relief of symptoms at 12 wk and 1 yr | Response rate: FMT 56% vs control 26% (P < 0.05); Good response predictor: women (69% vs 29%), patients with high baseline diversity, distinct composition (P < 0.05) 1 yr response FMT 21% vs control 6%; Second FMT restored response in 67% of patients with prior response | Unknown |
IBS-D: Diarrhea-predominant irritable bowel syndrome; IBS-M: Mixed-diarrhea-and-constipation irritable bowel syndrome; IBS-C: constipation-predominant irritable bowel syndrome; FMT: Fecal microbiota transplantation; IBS-SSS: Irritable bowel syndrome severity scoring system.
Donor selection
The outcomes of FMT in inflammatory bowel disease (IBD) have varied considerably between studies[20], which has been attributed to differences in the donor used[10,30]. A donor that produces a large response to FMT in IBD patients is called a superdonor[10]. Since it was not possible to predict who is a superdonor, the feces from several donors were pooled to increase the likelihood of patients receiving superdonor feces[31]. However, this approach was not successful, which was probably due to the dilution of the eventual superdonor feces resulting in an insufficient dose from the superdonor to the recipients[32].
Similar to IBD, discrepancies in the results of the RCTs of FMT in IBS could be mainly attributed to differences in the criteria used for donor selection (Table 1)[11,22-27]. An RCT performed by El-Salhy et al[11] that obtained good responses to FMT (Figure 1) established clinical criteria and the bacterial profile for selecting the superdonor. In contrast, the less-successful RCTs did not establish either clinical criteria or a bacterial profile for the donor[22-27] (Table 1). The stability of the donor intestinal bacterial composition over time is also an important factor to consider when selecting a superdonor[27].
Figure 1.
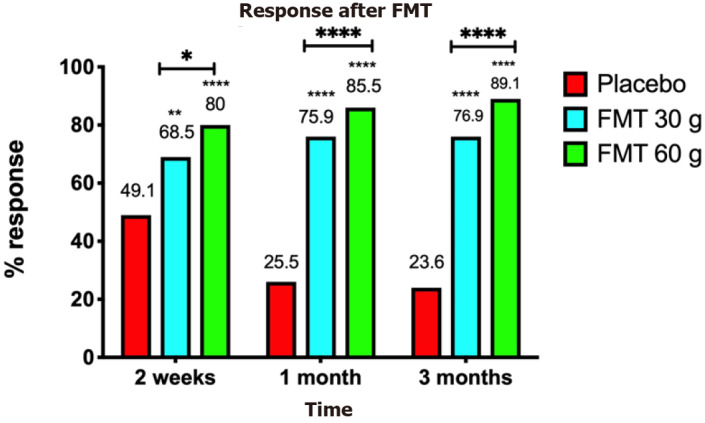
Responses of irritable bowel syndrome patients to placebo, 30-g fecal microbiota transplantation, and 60-g fecal microbiota transplantation at different intervals after transplantation. **P < 0.001; ****P < 0.0001 compared with placebo. **P < 0.001; ****P < 0.0001 for 30-g fecal microbiota transplantation (FMT) compared with 60-g FMT. Citation: El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020; 69: 859-867. Copyright @BMJ Publishing Group Ltd 2020. Published by BMJ Publishing Group Ltd[11].
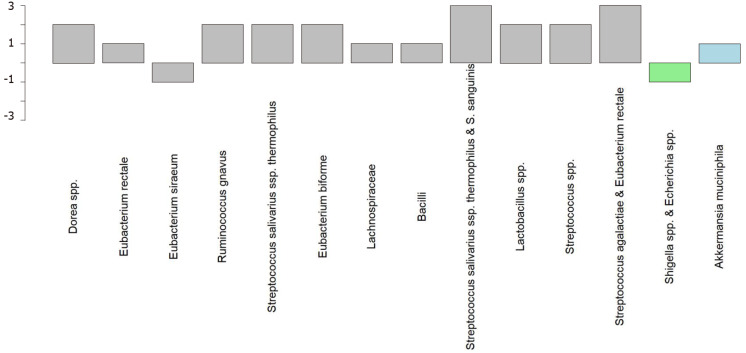
The clinical criteria used to select a superdonor have been based on the factors known to affect the intestinal microbiota (Table 2). Aging (> 50 years), smoking/smoking cessation, being born by cesarean section, consuming formula as a baby, frequent treatment with antibiotics, and regular intake of nonantibiotic drugs are known to reduce the bacterial diversity[33-42], whereas regular exercise combined with consuming a sport-specific diet are associated with a favorable intestinal microbiota[43-45]. The genetic composition also affects the intestinal microbiota, and hence the superdonor should not be a first-degree relative of any recipient[46,47]. Thus, the superdonor selected in the RCT by El-Salhy et al[11] was a healthy young male with a normal body mass index, born via a vaginal delivery, breastfed, and a nonsmoker, and did take any medication, had been treated only a few times with antibiotics, exercised regularly, and consumed a sport-specific diet that was richer in protein, fiber, minerals, and vitamins than average[48]. Furthermore, the superdonor was not related to any of the recipients[11]. The analysis of the fecal microbiota of this donor showed that he had a high microbial diversity (normobiotic), and his fecal bacterial composition deviated from the normal abundance of 165 healthy subjects in 14 of 39 tested bacteria markers (Figure 2)[11]. Twelve of these deviated bacteria were in the Firmicutes phylum, with one each in the Proteobacteria and Verrucomicrobia phyla[11]. This deviation included an increased abundance of favorable bacteria such as Streptococcus, Dorea, Lactobacillus, and Ruminococcaceae spp.[10,49-51]. The fecal bacterial composition of the superdonor was stable over the 18-mo period during which he donated his feces (Figure 3)[11].
Table 2.
Criteria of a super-donor and how the 7 published randomized clinical trials met them
|
Study
|
BMI sex
|
Age < 50 yr
|
Smoking
|
Vaginal delivery
|
Formula-fed
|
frequent treatment with antibiotics
|
regular intake of non-antibiotic drugs
|
regular exercise
|
consuming a sport-specific diet
|
first-degree relative
|
bacterial signature
|
stability of the bacterial composition over time
|
| El-Salhy et al[11], 2020 | Yes/male | Yes | No | Yes | No | No | No | Yes | Yes | No | YES | Yes |
| Johnsen et al[22], 2018 | Unknown/unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| Halkjær et al[23], 2018 | Yes/unknown | Yes | Unknown | Unknown | Unknown | Unknown | No | Unknown | Unknown | Unknown | Unknown | Unknown |
| Aroniadis et al[24], 2019 | Yes/unknown | Yes | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| Holster et al[25], 2019 | Unknown/unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| Lahtinen et al[26], 2020 | Yes/male | Yes | Unknown | Yes | Unknown | No | No | Unknown | No | Unknown | Unknown | Unknown |
| Holvoet et al[27], 2021 | Yes/male | Yes | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Yes |
BMI: Body mass index.
Figure 2.
Superdonor bacterial profile deviated from the expected normal abundance in 14 of the 39 bacteria markers. The deviating bacteria belong to the typical commensal bacteria species that do not contribute to dysbiosis. Twelve of these bacteria belong to the Firmicutes phylum (gray), one to the Proteobacteria phylum (light green), and one to the Verrucomicrobia phylum (light blue). Citation: El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020; 69: 859-867. Copyright @BMJ Publishing Group Ltd 2020. Published by BMJ Publishing Group Ltd[11].
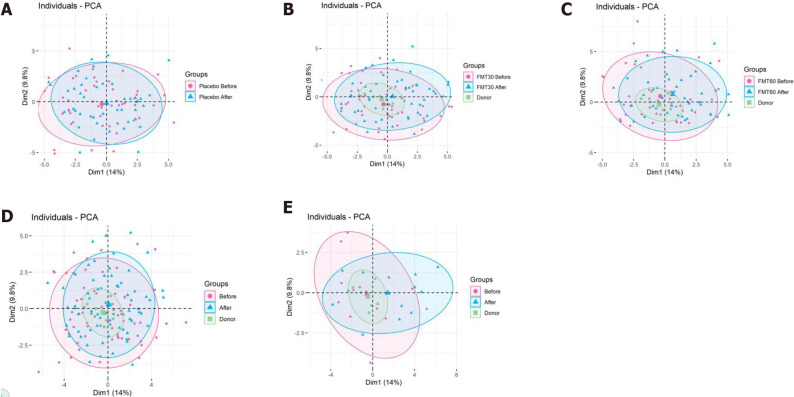
Figure 3.
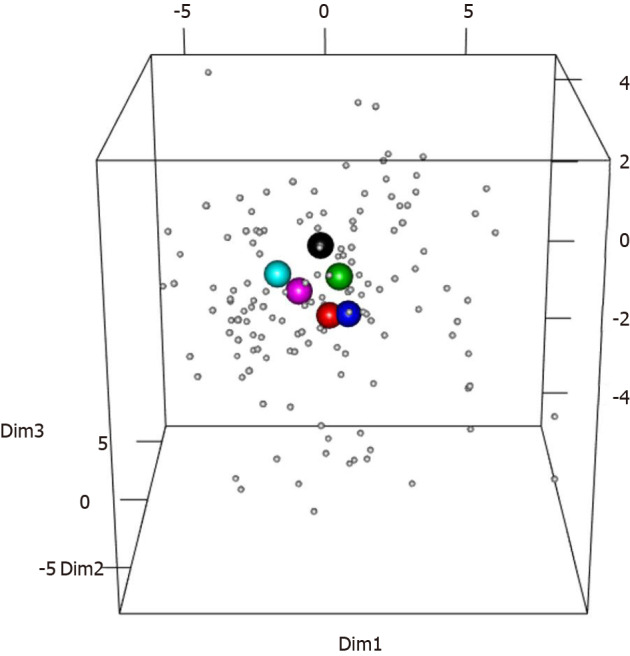
Scaled principal component analysis plot of fecal samples from the superdonor and patients before transplantation. The patient samples are indicated by small gray circles. The superdonor samples are indicated by the larger circles of different colors reflecting the sampling times: black, 3 mo; red, 6 mo; green, 9 mo; blue, 12 mo; light blue, 15 mo; and pink, 18 mo. All of the superdonor samples are grouped closely together and remain in very similar positions over time. Citation: El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020; 69: 859-867. Copyright @BMJ Publishing Group Ltd 2020. Published by BMJ Publishing Group Ltd[11].
Like in IBD, pooling the feces from several donors to ensure the presence of superdonor feces resulted in no response or only a transient improvement[22,23].
Patient inclusion
The patients included in RCTs of FMT for IBS have included subsets of IBS patients who do not represent the entire IBS population, and so caution should be exercised when generalizing the outcomes of these RCTs[11,22-27]. Four of the RCTs only included patients with the diarrhea-predominant IBS (IBS-D) and mixed-diarrhea-and-constipation IBS (IBS-M)[22,24,26,27], while the other three RCTs included three IBS subtypes: IBS-D, constipation-predominant IBS (IBS-C), and IBS-M[11,23,25]. Moreover, the patients included in the RCT of El-Salhy et al[11] had participated in a 2-d course related to living with IBS, and for at least the past 3 mo they had moderate-to-severe IBS symptoms despite adhering to a diet consistent with the National Institute for Health and Care Excellence (NICE)-modified diet. The RCT of Holster et al[25] included patients with low amounts of fecal butyrate-producing bacteria. Holvoet et al[27] only included refractory patients with severe bloating who had failed to respond to at least three conventional therapies for IBS.
Route of administration and dose of the fecal transplant
The fecal transplant can be administered either to the small intestine via the working channel of a gastroscope or a nasojejunal probe, or to the large intestine via the working channel of a colonoscope (Figures 4 and 5)[11,22,25-27]. Administering the fecal transplant to either the small or large intestine seems to be effective[11,22,25-27]. However, a placebo effect was evident in 43%-44% and 23.6%-26% of the patients that received the fecal transplant into the large and small intestine, respectively[11,22,25-27]. This placebo effect might have been greater in patients that received fecal transplant into the colon due to the favorable effect of the bowel preparation needed for a colonoscopy on IBS symptoms[52].
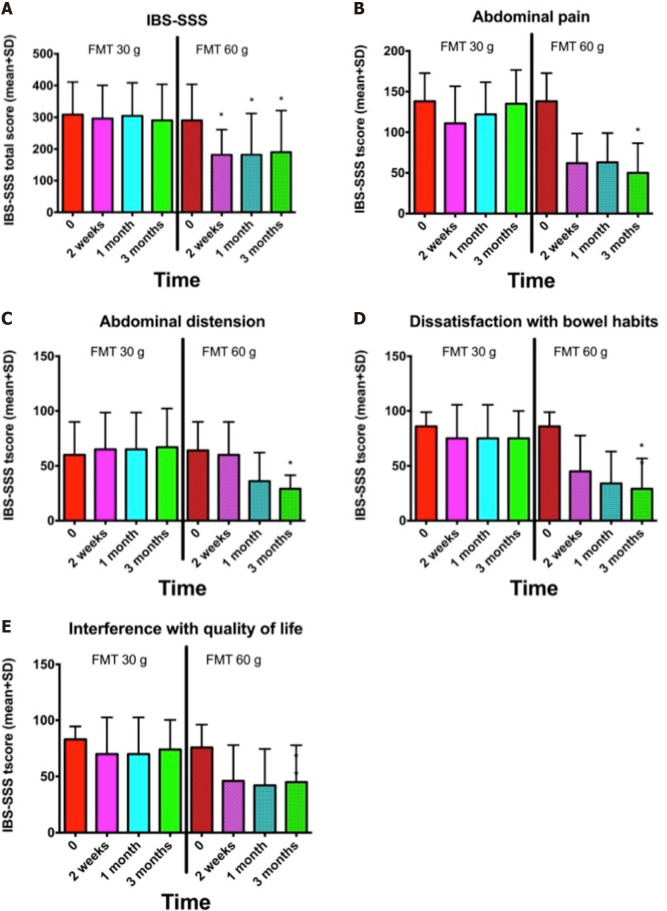
Figure 4.
Nonresponders to 30-g donor`s fecal transplant were retransplanted with 60-g donor´s transplant. A-E: Irritable bowel syndrome-severity scoring system (SSS) total score (A) and IBS-SSS scores for abdominal pain (B), abdominal distension (C), dissatisfaction with bowel habits (D), and interference with quality of life (E) at different intervals after receiving 30-g and 60-g fecal microbiota transplantation. *P < 0.05 compared with baseline. Citation: El-Salhy M, Hausken T, Hatlebakk JG. Increasing the Dose and/or Repeating Faecal Microbiota Transplantation (FMT) Increases the Response in Patients with Irritable Bowel Syndrome (IBS). Nutrients 2019; 11 [PMID: 31238507 DOI: 10.3390/nu11061415]54].
Figure 5.
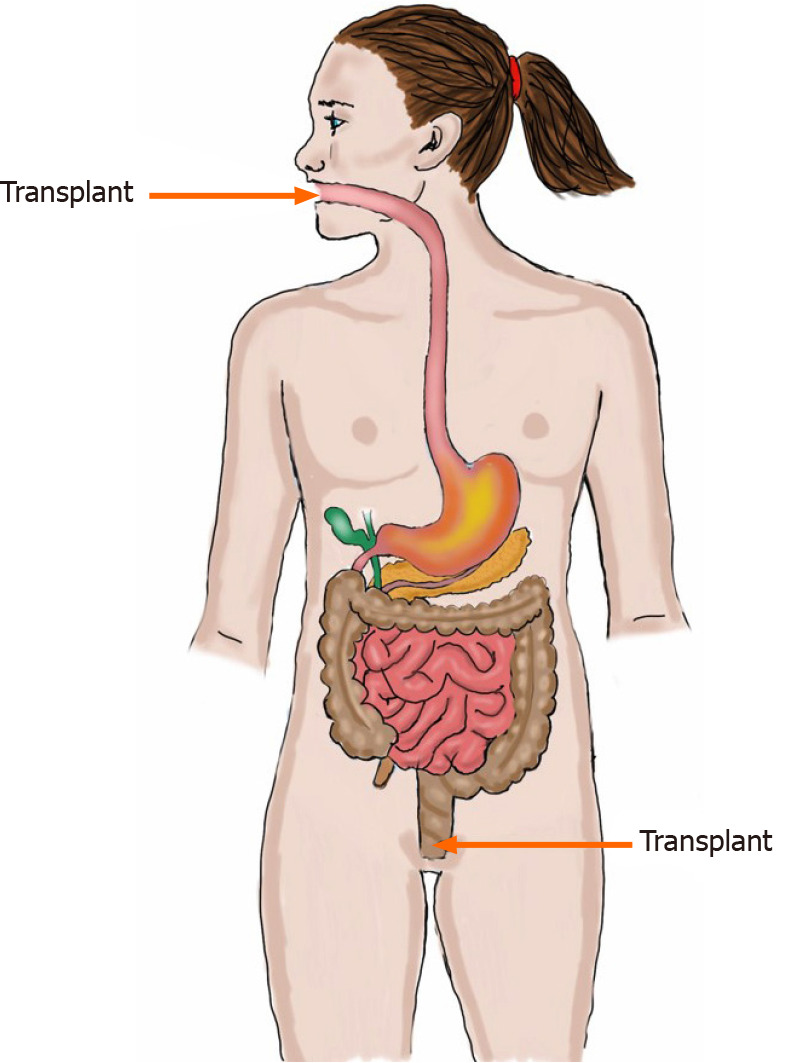
Fecal transplant can be administered to the small intestine (distal duodenum) or to the large intestine (colon).
While administering fecal transplants via capsule ingestion has been effective in CDI, it was unsuccessful in IBS[23,24,53]. This is could be due to the selection of the donor, a low transplant dose, or pooling of the donors rather than the mode of administration[23,24].
The dose of the fecal transplant seems to significantly affect the outcome of FMT, with a dose–dependent response appearing to be present (Figure 1)[11]. This was confirmed by 70% of patients that did not respond to 30-g FMT responding to 60-g FMT (Figure 6)[54]. All but two of the RCTs of FMT for IBS used a dose of at least 30 g[11,22,23,25,26]; in the remaining two studies the dose was either lower than 30 g or not specified[24,27]. Further investigations are needed to evaluate the efficacy single vs repeated transplantation.
Figure 6.
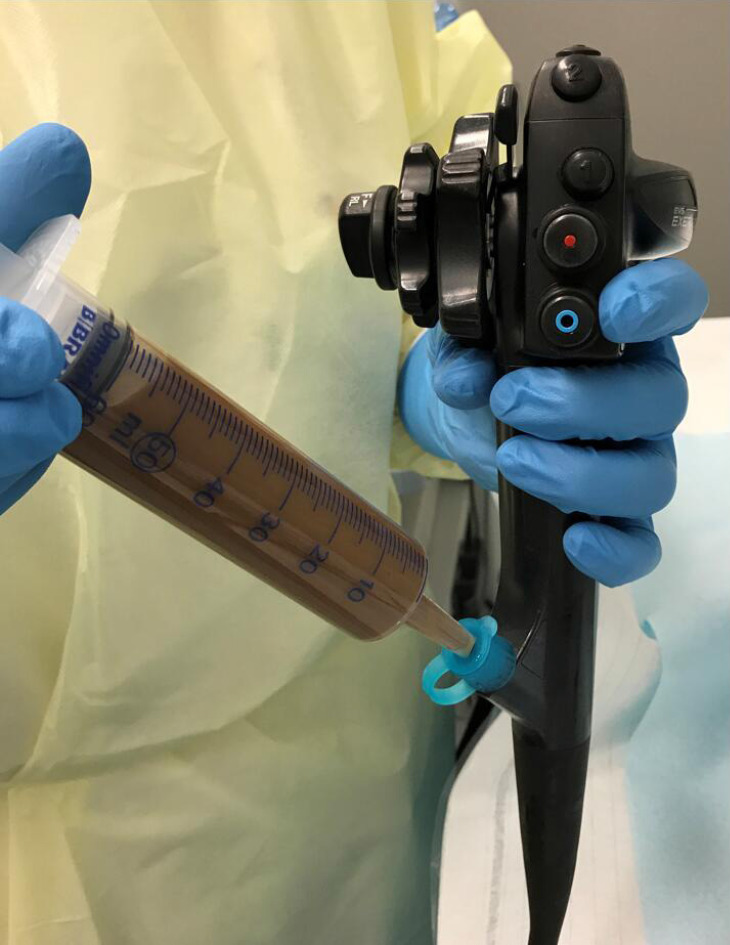
Although the fecal transplant can be administered to the small intestine via a nasojejunal probe and to the colon via an enema, administering a fecal transplant via the working channel of a gastroscope or colonoscope is faster and more acceptable to patients.
CHANGES IN BACTERIAL AND FERMENTATION PROFILES AFTER FMT
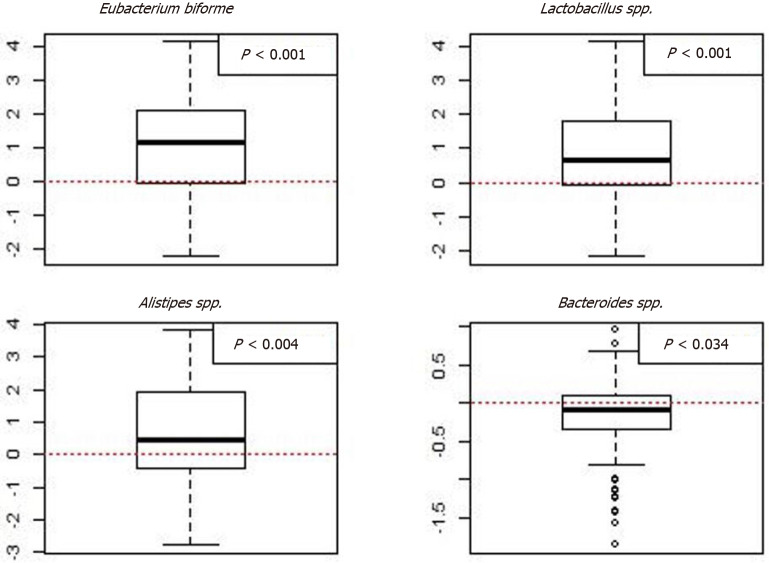
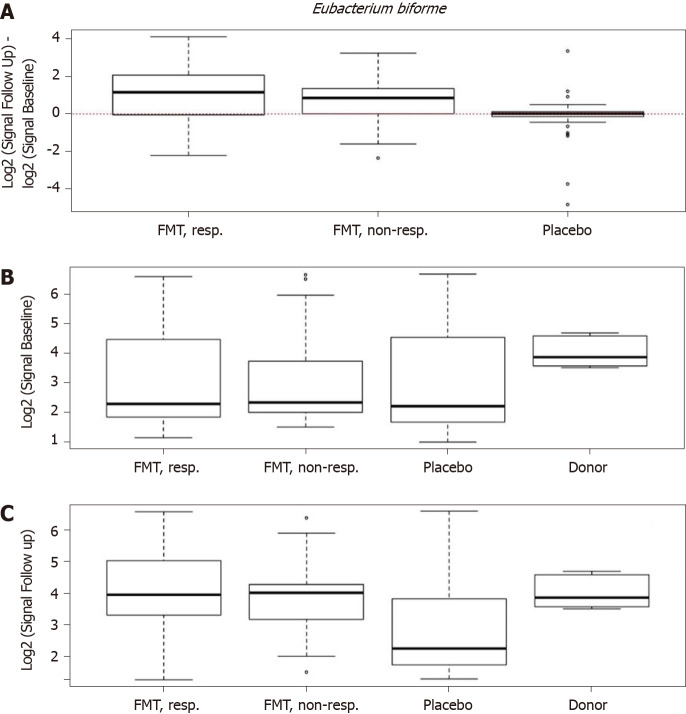
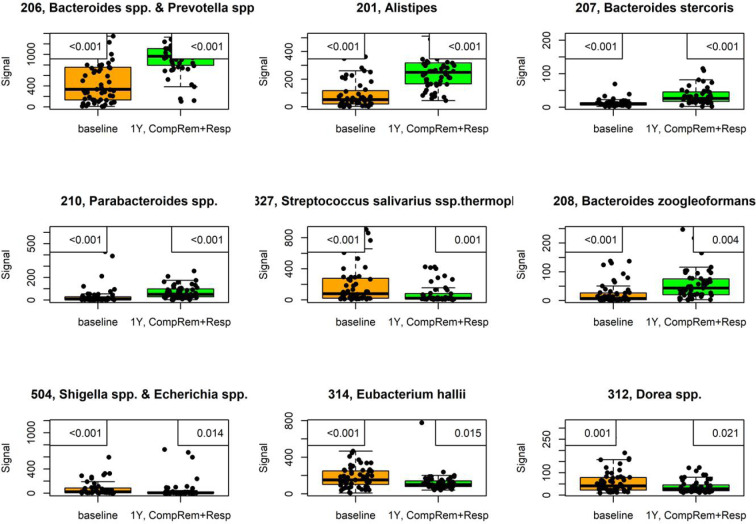
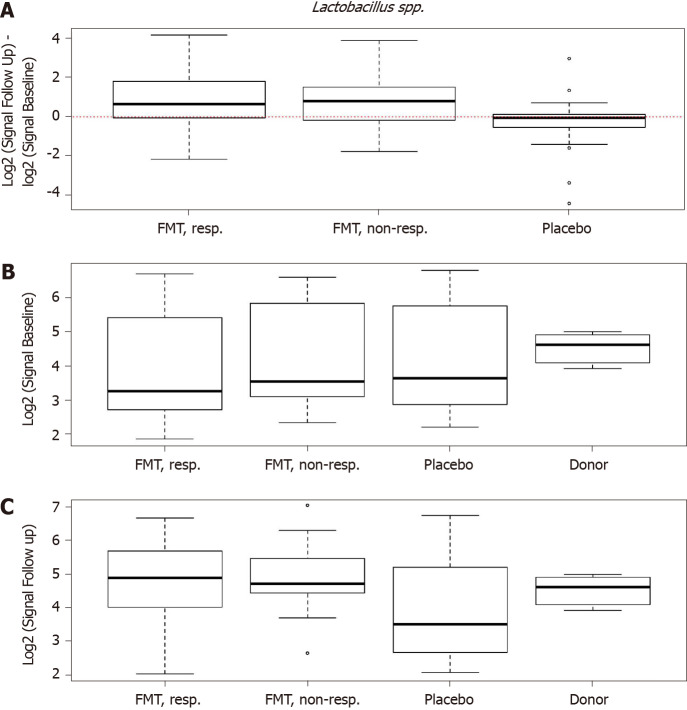
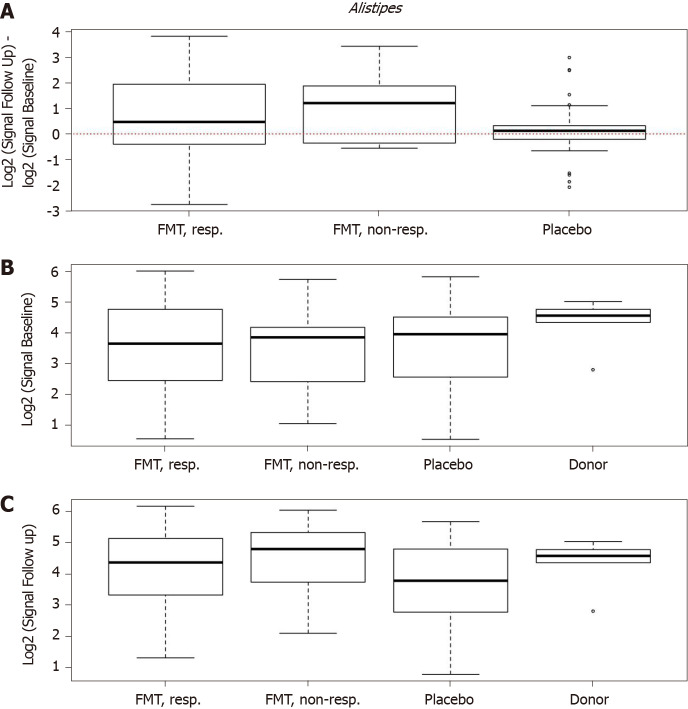
In the RCT of El-Salhy et al[11,55] in which FMT was most effective, the severity of dysbiosis did not differ significantly between the placebo and active treated groups after 1 mo, but it was reduced significantly after 1 year. One month after FMT, the bacterial profile changed significantly in the active treated groups compared with the placebo group (Figure 7)[11]. Higher signals for Eubacterium biforme, Lactobacillus spp., and Alistipes spp., and lower signals for Bacteroides spp. were observed at 1 mo after FMT in the active treated groups (Figure 8) but not in the placebo group (Figures 9–12)[11]. The IBS severity scoring system (IBS-SSS) score was significantly correlated with the signals of Lactobacillus spp. (P = 0.002, r = –0.3) and Alistipes spp. (P = 0.001, r = –0.3) but not with those of Eubacterium biforme(P = 0.754, r = 0.03) or Bacteroides spp. (P = 0.458, r = 0.06)[11]. The signals of nine bacteria had changed at 1 year after FMT (Figure 13)(unpublished data): Whereas the signals of Bacteroides spp., Prevotella spp., Alistipes spp., Bacteroides stercoris, Parabacteroides spp., and Bacteroides zoogleoformans were significantly higher than those at the baseline, the signals of Streptococcus salivarius ssp. thermophilus, Shigella spp., Escherichia spp., Eubacterium hallii, and Dorea spp. were significantly lower than those at the baseline. The signals of the following six of these bacteria were correlated with IBS-SSS total scores: Bacteroides spp. And Prevotella spp. (P < 0.0001, r = -0.4), Alistipes spp. (P < 0.001, r = -0.3), Bacteroides stercoris and Streptococcus salivarius ssp. thermophilus (P < 0.0001, r = 0.3), Bacteroides zoogleoformans (P < 0.002, r = -0.3), and Eubacterium hallii (P < 0.04, r = -0.2) (unpublished data). Changes in the bacterial profiles in IBS patients that received FMT have been recorded, but which bacteria had changed was not described in detail[23,25-27].
Figure 7.
Scaled principal component analysis plot of fecal samples before and 1 mo after transplantation for placebo, 30-g and 60-g fecal microbiota transplantation, responders and nonresponders. A: Placebo; B: 30-g fecal microbiota transplantation (FMT); C: 60-g FMT; D: Responders; E: Nonresponders. Fecal samples before and after transplantation are indicated by pink circles and blue triangles, respectively. The ellipses cover 80% of the samples within a group. Citation: El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020; 69: 859-867. Copyright @BMJ Publishing Group Ltd 2020. Published by BMJ Publishing Group Ltd[11].
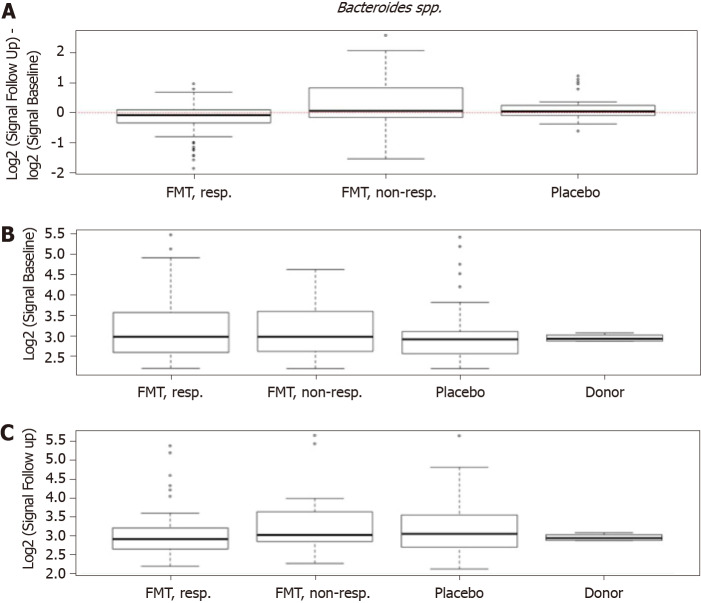
Figure 8.
Box plots of the logarithm of the difference in signals between baseline and 1 mo after fecal microbiota transplantation for the responders in both the 30-g and 60-g fecal microbiota transplantation groups. Red stippled line indicates 0, where no change occurred between before and after fecal microbiota transplantation (FMT). Signals after FMT in responders were higher for Eubacterium biforme, Lactobacillus spp., and Alistipes spp., and lower for Bacteroides spp. Citation: El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020; 69: 859-867. Copyright @BMJ Publishing Group Ltd 2020. Published by BMJ Publishing Group Ltd[11].
Figure 9.
Differences in signals for Eubacterium biforme in the responder, nonresponder, and placebo groups between before and 1 mo after transplantation. There were significant changes in the signal levels in responders (P < 0.001). The nonresponders also exhibited increased signal levels, though the changes were smaller than those in the responders. A: The signal level did not change in the placebo group; B: Signal levels at baseline were similar in the responder, nonresponder, and placebo groups, but they were higher for the superdonor; C: The signal levels in the responders and nonresponders became as high as that for the superdonor after fecal microbiota transplantation, while that in the placebo group did not change.
Figure 13.
Signals of nine bacteria had changed at 1 year after fecal microbiota transplantation in irritable bowel syndrome patients that either maintained a response or experienced complete remission. P values are in the upper-left corner, with corrected P values in the upper-right corner. This figure is based on unpublished data from El-Salhy et al.
Figure 10.
Differences in signals for Lactobacillus spp. in the responder, nonresponder, and placebo groups between before and 1 mo after transplantation. There were significant changes in the signal levels in responders (P < 0.001). A: The nonresponders exhibited similar signal levels to those in the responders, while there was no change in the placebo group; B: The signal levels in the responder, nonresponder, and placebo groups were similar, but it was higher for the superdonor; C: After transplantation, the signal levels in responders and nonresponders became similar to that for the superdonor, while that in the placebo group did not differ from the baselin. Citation: El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020; 69: 859-867. Copyright @BMJ Publishing Group Ltd 2020. Published by BMJ Publishing Group Ltd[11].
Figure 11.
Changes in signals for Alistipes spp. in responders, nonresponders, and placebo groups as well as in the donor. A: Differences in signals for Alistipes spp. between before and 1 mo after transplantation in the responder, nonresponder, and placebo groups; B: There were significant changes in the signals level in responders (P = 0.004). The signal levels also changed in nonresponders, but they did not change in the placebo group. Signal levels at baseline in the responder, nonresponder, and placebo groups as well as the superdonor; C: The baseline signal levels were similar in the responder, nonresponder, and placebo groups, while that for the superdonor was higher. Signal levels in the responder, nonresponder, and placebo groups and the superdonor after transplantation. The signal levels were similar in responders, nonresponders and the superdonor, while it was lower in the placebo group. Citation: El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020; 69: 859-867. Copyright @BMJ Publishing Group Ltd 2020. Published by BMJ Publishing Group Ltd[11].
Figure 12.
Changes in signals for Bacteroides spp. in responders, nonresponders, and placebo groups as well as in the donor. A: Differences in signals for Bacteroides spp. between before and 1 mo after fecal microbiota transplantation in the responder, nonresponder, and placebo groups; B: There were significant changes in the signal levels in responders (P < 0.034). The nonresponders showed similar changes, while there were no changes observed in the placebo group. Signal levels at baseline in the responder, nonresponder, and placebo groups and for the superdonor; C: The baseline signal levels were similar in the responder, nonresponder, and placebo groups, and both higher and lower than those for the superdonor. Signal levels in the same groups after transplantation. Citation: El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020; 69: 859-867. Copyright @BMJ Publishing Group Ltd 2020. Published by BMJ Publishing Group Ltd[11].
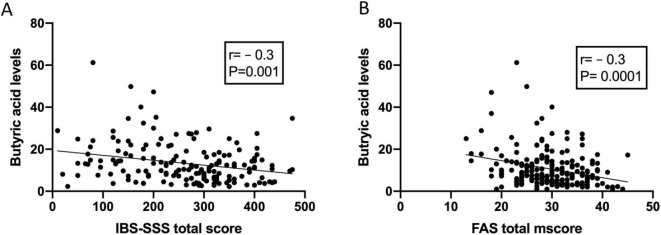
The main products of the bacterial fermentation of intestinal undigested and unabsorbed carbohydrates are short-chain fatty acids (SCFAs)[55]. It has been reported that the fecal level of propionic acid was significantly higher in IBS patients[13]. Moreover, the level of butyric acid has been found to be lower in IBS-C patients and higher in IBS-D patients than in healthy subjects[13]. The levels of total SCFAs at 1 mo after FMT were significantly higher in IBS patients that received 60-g FMT but not in those that received 30-g FMT[55]. The levels of butyric acid at 1 mo after FMT were also significantly higher in IBS patients that received either 30-g or 60-g FMT[55]. There were no significant differences in the levels of total SCFAs and in level of individual SCFAs in the placebo group at 1 mo after FMT[55]. The butyric acid level was inversely correlated with the scores on the IBS-SSS and fatigue assessment scale (Figure 14)[55].
Figure 14.
The correlation between butyric acid levels and IBS symptoms and fatigue 1 mo after fecal transplation. A and B: Correlations between butyric acid levels and irritable bowel syndrome-severity scoring system total scores (A) and fatigue assessment total score (B). Citation: El-Salhy M, Hausken T, Hatlebakk JG. Increasing the Dose and/or Repeating Faecal Microbiota Transplantation (FMT) Increases the Response in Patients with Irritable Bowel Syndrome (IBS). El-Salhy M, Valeur J, Hausken T, Gunnar Hatlebakk J. Changes in fecal short-chain fatty acids following fecal microbiota transplantation in patients with irritable bowel syndrome. Neurogastroenterol Motil 2021; 33: e13983 [PMID: 32945066 DOI: 10.1111/nmo.13983][55].
The fecal levels of total SCFAs and butyric acid remained elevated in the active treated group with FMT at 1 year after FMT (unpublished data). Furthermore, the fecal levels of isobutyric and isovaleric acids were significantly higher while that of acetic acid was significantly lower (unpublished data).
DIFFERENCES IN RESPONSES BETWEEN IBS SUBTYPES AND SEX
The response to FMT did not differ between the IBS-D, IBS-C, and IBS-M subtypes of IBS[11], whereas it was not clear whether there was a difference between females and males. In the subgroup of IBS-D and IBS-M patients with refractory IBS and severe bloating who had not responded to at least three conventional therapies for IBS, the response to FMT was better for females than for males[27]. On the other hand, the response to FMT did not differ between female and male IBS patients with moderate-to-severe symptoms belonging to the IBS-D, IBS-C, and IBS-M subtypes who had previously not responded to the NICE-modified diet[56].
SAFETY OF FMT
A recently published consensus report from a multidisciplinary United European Gastroenterology working group did not classify voluntarily donated material (feces) as a drug. Instead, it was recommended to be collected, handled, and used according to the standards defined by the EU Commission in EU Tissue and Cells Directive 2004/23/EC[21,57].
FMT appears to be safe, with limited side effects[58,59]. The short-term adverse events for FMT in IBS patients have been reported to be mild, self-limiting, and occurring during the first few days following transplantation: Abdominal pain, cramping, tenderness, diarrhea, and constipation[11,22-27]. However, infectious complications have been described in two patients that received FMT for diseases other than IBS, which resulted in one fatality[60,61]. While these patients were old, severely ill, and immunocompromised, such events raised questions about safety issues regarding FMT for IBS, especially given that IBS is considered to be a benign gastrointestinal condition[3,62,63]. It has therefore been suggested that the screening of FMT donors should include testing of their feces for extended-spectrum-beta-lactase-producing E. coli and severe acute respiratory syndrome coronavirus 2. This would reduce the risks of infection and restrict the selection of IBS patients for FMT to those who are not immunocompromised and do not have systemic disease or severe illness[3].
No adverse events were observed 1-year after FMT for IBS patients (unpublished data). However, long-term follow-ups of patients that received FMT because of CDI raised some concerns about weight gain, the development or worsening of IBD, cancer, autoimmune diseases, allergies, and neurological diseases[64].
POSSIBLE MECHANISMS UNDERLYING THE EFFECTS OF FMT
The enteroendocrine cells and fecal SCFAs in IBS patients differ from those of healthy subjects, and these differences are believed to play a central role in the pathophysiology of IBS[2,65-70]. Although it is early to speculate about the mechanisms underlying the effects of FMT, there are data suggesting that the improvement in IBS symptoms by FMT is caused by changes in the enteroendocrine cells and in SCFAs.
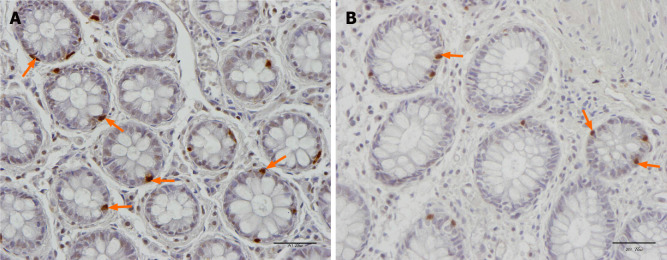
Most of the body serotonin (95%) is localized to the gastrointestinal tract, of which only 10% is present in the neurons of the enteric nervous system and the rest is in the serotonin-containing enterochromaffin (EC) cells that are scattered between the epithelial cells lining the gastrointestinal lumen[71-74]. Serotonin plays an important role in gastrointestinal motility by inhibiting gastric emptying and stimulating colonic motility, and accelerating transit through the small and large intestines. Serotonin also activates the submucosal sensory branch of the enteric nervous system that conveys sensation from the gut to the central nervous system[70]. When serotonin exerts its effects at serotonin receptors, it is transported by the serotoninselective reuptake transporter (SERT) into intestinal epithelial cells, where it is degraded[75,76]. The epithelial cells lining the luminal surface of the intestine express SERT[70,77], and a reduction in SERT results in impaired intracellular uptake of serotonin and its degradation in the intestinal epithelial cells[78,79]. In IBS patients, the density of EC cells in the colon and the SERT immune-intensity in the large intestine have been reported to be lower than those in healthy subjects (Figures 15 and 16)[80-82]. Several bacteria including Corynebacterium, Streptococcus, and Enterococcus spp. produce serotonin and indigenous spore-forming bacteria from the microbiota of mice and humans that promote serotonin biosynthesis by colonic EC cells[83,84]. Furthermore, Clostridium ramosum regulates the release of serotonin from EC cells[85]. It is possible that the changes in the intestinal bacterial composition induced by FMT affect the serotonin-regulating system.
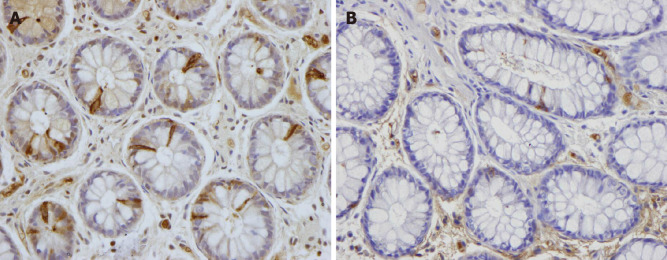
Figure 15.
Serotonin-immunoreactive cells (arrows) in the colon of a healthy subject and in a patient with irritable bowel syndrome. A: Healthy subject; B: Patient with irritable bowel syndrome.
Figure 16.
Difference in serotonin selective reuptake transporter immunoreactivity between a healthy subject and a patient with irritable bowel syndrome. A and B: Serotonin selective reuptake transporter immunoreactivity in the cytoplasm of the epithelial cells lining the rectal lumen (arrows) of a healthy subject (A) and of an irritable bowel syndrome patient (B).
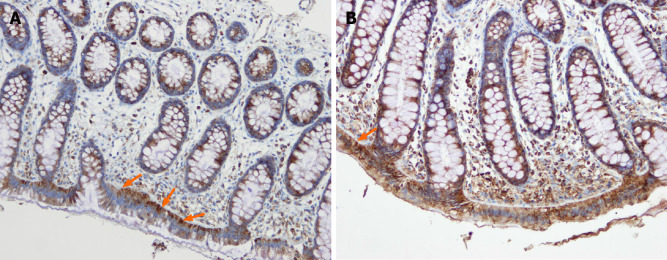
Peptide YY (PYY) occurs in enteroendocrine cells of the ileum, colon, and rectum[86,87]. PYY binds to and activates receptors Y1 and Y2, which are localized to the epithelial cells lining the intestinal lumen and in the submucosal and myenteric plexus neurons of the small intestine and colon[88-100]. PYY acts as a mediator of the ileal brake and stimulates the absorption of water and electrolytes in the large intestine[87,101,102]. The density of colonic PYY cells is reduced in IBS patients (Figure 17)[80-82]. The total levels of fecal SCFAs increased significantly in IBS patients at 1 mo after FMT and remained higher than those at the baseline at 1 year after transplantation(unpublished data)[55]. Intestinal SCFAs increase the secretion of peptide PYY by up-regulating the gene expression of PYY[102-105]. It is likely that the improvement in IBS symptoms following FMT is due to the effect of SCFAs on PYY secretion.
Figure 17.
Peptide YY immunoreactivity (arrows) in the colon of a healthy subject and in a patient with irritable bowel syndrome. A: Healthy subject; B: Patient with irritable bowel syndrome.
In the patients that received FMT, the fecal levels of butyric acid increased at 1 mo after FMT and remained elevated at 1 year after transplantation(unpublished data)[56]. Butyrate provides colonic epithelial cells with energy[105], modulates the immune response and oxidative stress, and decreases intestinal motility and the permeability of intestinal cells[87]. Butyrate also modulates colonic hypersensitivity, and an intake of butyrate was found to reduce the abdominal pain in patients with IBS[106-108]. An increase in butyric acid could be one of the factors underlying the improvement seen after FMT.
The fecal level of the straight SCFA acetic acid was reduced at 1 year after FMT(unpublished data). This reduction could be significant since acetic acid has been found to induce visceral hypersensitivity in rodents[109]. The levels of the branched SCFAs isobutyric and isovaleric acids were significantly increased at 1 year after FMT(unpublished data). These increases indicate a shift in the microbial metabolism from a saccharolytic to a proteolytic fermentation pattern, which could be of pathophysiological relevance[110]. It is particularly interesting that similar changes in the profiles of isobutyric and isovaleric acids have been found in patients with IBS following adherence to a low-FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet[111].
CONCLUSION
FMT is a longstanding Chinese treatment applied to both gastrointestinal and nongastrointestinal diseases[18], and is now also a promising treatment for IBS patients since it improves the IBS symptoms, fatigue, and quality of life in about 90% of patients in a RCT[11]. However, several questions remain to be answered, and further investigations are needed before FMT can be applied in everyday clinical practice. The criteria to apply when selecting an effective (superdonor) for FMT remain unclear, as do the optimal dose, administration route, and frequency of treatment. Moreover, it is not clear whether FMT is effective for all IBS patients, or whether it should be restricted to certain subsets of IBS patients. There is also some concern regarding the long-term side effects of FMT[21].
There is an exciting task in front of us to modernize an effective treatment for IBS that was first used more than a thousand years ago.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: February 5, 2021
First decision: March 29, 2021
Article in press: May 22, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Norway
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu LH S-Editor: Zhang H L-Editor: A P-Editor: Liu JH
Contributor Information
Magdy El-Salhy, Department of Medicine, Stord Helse Fonna Hospital and University of Bergen, Stord 5416, Norway. magdy.elsalhy@sklbb.no.
Tanisa Patcharatrakul, Department of Medicine, King Chulalongkorn Memorial Hospital and Center of Excellence in Neurogastroenterology and Motility, Chulalongkorn University, Bangkok 10330, Thailand.
Sutep Gonlachanvit, Department of Medicine, King Chulalongkorn Memorial Hospital and Center of Excellence in Neurogastroenterology and Motility, Chulalongkorn University, Bangkok 10330, Thailand.
References
- 1.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71–80. doi: 10.2147/CLEP.S40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Salhy M. Recent developments in the pathophysiology of irritable bowel syndrome. World J Gastroenterol. 2015;21:7621–7636. doi: 10.3748/wjg.v21.i25.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Salhy M. FMT in IBS: how cautious should we be? Gut. 2021;70:626–628. doi: 10.1136/gutjnl-2020-322038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol. 2011;62:591–599. [PubMed] [Google Scholar]
- 5.El-Salhy M, Gilja OH, Hatlebakk JG. Overlapping of irritable bowel syndrome with erosive esophagitis and the performance of Rome criteria in diagnosing IBS in a clinical setting. Mol Med Rep. 2019;20:787–794. doi: 10.3892/mmr.2019.10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong PP, Chin VK, Looi CY, Wong WF, Madhavan P, Yong VC. The Microbiome and Irritable Bowel Syndrome - A Review on the Pathophysiology, Current Research and Future Therapy. Front Microbiol. 2019;10:1136. doi: 10.3389/fmicb.2019.01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hugon P, Dufour JC, Colson P, Fournier PE, Sallah K, Raoult D. A comprehensive repertoire of prokaryotic species identified in human beings. Lancet Infect Dis. 2015;15:1211–1219. doi: 10.1016/S1473-3099(15)00293-5. [DOI] [PubMed] [Google Scholar]
- 9.Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R, Gordon JI. Evolution of mammals and their gut microbes. Science. 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson BC, Vatanen T, Cutfield WS, O'Sullivan JM. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front Cell Infect Microbiol. 2019;9:2. doi: 10.3389/fcimb.2019.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut. 2020;69:859–867. doi: 10.1136/gutjnl-2019-319630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kriss M, Hazleton KZ, Nusbacher NM, Martin CG, Lozupone CA. Low diversity gut microbiota dysbiosis: drivers, functional implications and recovery. Curr Opin Microbiol. 2018;44:34–40. doi: 10.1016/j.mib.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Salhy M, Mazzawi T. Fecal microbiota transplantation for managing irritable bowel syndrome. Expert Rev Gastroenterol Hepatol. 2018;12:439–445. doi: 10.1080/17474124.2018.1447380. [DOI] [PubMed] [Google Scholar]
- 14.Casén C, Vebø HC, Sekelja M, Hegge FT, Karlsson MK, Ciemniejewska E, Dzankovic S, Frøyland C, Nestestog R, Engstrand L, Munkholm P, Nielsen OH, Rogler G, Simrén M, Öhman L, Vatn MH, Rudi K. Deviations in human gut microbiota: a novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment Pharmacol Ther. 2015;42:71–83. doi: 10.1111/apt.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enck P, Mazurak N. Dysbiosis in Functional Bowel Disorders. Ann Nutr Metab. 2018;72:296–306. doi: 10.1159/000488773. [DOI] [PubMed] [Google Scholar]
- 16.Pozuelo M, Panda S, Santiago A, Mendez S, Accarino A, Santos J, Guarner F, Azpiroz F, Manichanh C. Reduction of butyrate- and methane-producing microorganisms in patients with Irritable Bowel Syndrome. Sci Rep. 2015;5:12693. doi: 10.1038/srep12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu HN, Wu H, Chen YZ, Chen YJ, Shen XZ, Liu TT. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig Liver Dis. 2017;49:331–337. doi: 10.1016/j.dld.2017.01.142. [DOI] [PubMed] [Google Scholar]
- 18.Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1755; author reply p.1755–1755; author reply p.1756. doi: 10.1038/ajg.2012.251. [DOI] [PubMed] [Google Scholar]
- 19.Liubakka A, Vaughn BP. Clostridium difficile Infection and Fecal Microbiota Transplant. AACN Adv Crit Care. 2016;27:324–337. doi: 10.4037/aacnacc2016703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green JE, Davis JA, Berk M, Hair C, Loughman A, Castle D, Athan E, Nierenberg AA, Cryan JF, Jacka F, Marx W. Efficacy and safety of fecal microbiota transplantation for the treatment of diseases other than Clostridium difficile infection: a systematic review and meta-analysis. Gut Microbes. 2020;12:1–25. doi: 10.1080/19490976.2020.1854640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller JJ, Ooijevaar RE, Hvas CL, Terveer EM, Lieberknecht SC, Högenauer C, Arkkila P, Sokol H, Gridnyev O, Mégraud F, Kump PK, Nakov R, Goldenberg SD, Satokari R, Tkatch S, Sanguinetti M, Cammarota G, Dorofeev A, Gubska O, Laniro G, Mattila E, Arasaradnam RP, Sarin SK, Sood A, Putignani L, Alric L, Baunwall SMD, Kupcinskas J, Link A, Goorhuis AG, Verspaget HW, Ponsioen C, Hold GL, Tilg H, Kassam Z, Kuijper EJ, Gasbarrini A, Mulder CJJ, Williams HRT, Vehreschild MJGT. A standardised model for stool banking for faecal microbiota transplantation: a consensus report from a multidisciplinary UEG working group. United European Gastroenterol J. 2021;9:229–247. doi: 10.1177/2050640620967898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnsen PH, Hilpüsch F, Cavanagh JP, Leikanger IS, Kolstad C, Valle PC, Goll R. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. 2018;3:17–24. doi: 10.1016/S2468-1253(17)30338-2. [DOI] [PubMed] [Google Scholar]
- 23.Halkjær SI, Christensen AH, Lo BZS, Browne PD, Günther S, Hansen LH, Petersen AM. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut. 2018;67:2107–2115. doi: 10.1136/gutjnl-2018-316434. [DOI] [PubMed] [Google Scholar]
- 24.Aroniadis OC, Brandt LJ, Oneto C, Feuerstadt P, Sherman A, Wolkoff AW, Kassam Z, Sadovsky RG, Elliott RJ, Budree S, Kim M, Keller MJ. Faecal microbiota transplantation for diarrhoea-predominant irritable bowel syndrome: a double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2019;4:675–685. doi: 10.1016/S2468-1253(19)30198-0. [DOI] [PubMed] [Google Scholar]
- 25.Holster S, Lindqvist CM, Repsilber D, Salonen A, de Vos WM, König J, Brummer RJ. The Effect of Allogenic Versus Autologous Fecal Microbiota Transfer on Symptoms, Visceral Perception and Fecal and Mucosal Microbiota in Irritable Bowel Syndrome: A Randomized Controlled Study. Clin Transl Gastroenterol. 2019;10:e00034. doi: 10.14309/ctg.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahtinen P, Jalanka J, Hartikainen A, Mattila E, Hillilä M, Punkkinen J, Koskenpato J, Anttila VJ, Tillonen J, Satokari R, Arkkila P. Randomised clinical trial: faecal microbiota transplantation versus autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment Pharmacol Ther. 2020;51:1321–1331. doi: 10.1111/apt.15740. [DOI] [PubMed] [Google Scholar]
- 27.Holvoet T, Joossens M, Vázquez-Castellanos JF, Christiaens E, Heyerick L, Boelens J, Verhasselt B, van Vlierberghe H, De Vos M, Raes J, De Looze D. Fecal Microbiota Transplantation Reduces Symptoms in Some Patients With Irritable Bowel Syndrome With Predominant Abdominal Bloating: Short- and Long-term Results From a Placebo-Controlled Randomized Trial. Gastroenterology 2021; 160: 145-157. :e8. doi: 10.1053/j.gastro.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Segal JP, Mullish BH, Quraishi MN, Iqbal TH. Letter: faecal microbiota transplantation for IBS. Aliment Pharmacol Ther. 2020;52:556–557. doi: 10.1111/apt.15824. [DOI] [PubMed] [Google Scholar]
- 29.Ianiro G, Porcari S, Ford AC, Gasbarrini A, Cammarota G. Letter: faecal microbiota transplantation for irritable bowel syndrome-room for improvement. Aliment Pharmacol Ther. 2020;52:923–924. doi: 10.1111/apt.15923. [DOI] [PubMed] [Google Scholar]
- 30.Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, Armstrong D, Marshall JK, Kassam Z, Reinisch W, Lee CH. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015; 149: 102-109. :e6. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Kazerouni A, Wein LM. Exploring the Efficacy of Pooled Stools in Fecal Microbiota Transplantation for Microbiota-Associated Chronic Diseases. PLoS One. 2017;12:e0163956. doi: 10.1371/journal.pone.0163956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, Leong RWL, Connor S, Ng W, Paramsothy R, Xuan W, Lin E, Mitchell HM, Borody TJ. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389:1218–1228. doi: 10.1016/S0140-6736(17)30182-4. [DOI] [PubMed] [Google Scholar]
- 33.Capurso G, Lahner E. The interaction between smoking, alcohol and the gut microbiome. Best Pract Res Clin Gastroenterol. 2017;31:579–588. doi: 10.1016/j.bpg.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Biedermann L, Brülisauer K, Zeitz J, Frei P, Scharl M, Vavricka SR, Fried M, Loessner MJ, Rogler G, Schuppler M. Smoking cessation alters intestinal microbiota: insights from quantitative investigations on human fecal samples using FISH. Inflamm Bowel Dis. 2014;20:1496–1501. doi: 10.1097/MIB.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 35.Korpela K, Dikareva E, Hanski E, Kolho KL, de Vos WM, Salonen A. Cohort profile: Finnish Health and Early Life Microbiota (HELMi) longitudinal birth cohort. BMJ Open. 2019;9:e028500. doi: 10.1136/bmjopen-2018-028500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeung OY, Ng YF, Chiou J, Wong MS. Pilot Study to Determine the Gut Microbiota of Hong Kong Infants Fed with Breast-milk And/or Infant Formula (P11-101-19) Curr Develop Nutr. 2019;9:3 (Suppl 1). [Google Scholar]
- 37.Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, Björkstén B, Engstrand L, Andersson AF. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63:559–566. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 38.Rutayisire E, Huang K, Liu Y, Tao F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants' life: a systematic review. BMC Gastroenterol. 2016;16:86. doi: 10.1186/s12876-016-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ianiro G, Tilg H, Gasbarrini A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut. 2016;65:1906–1915. doi: 10.1136/gutjnl-2016-312297. [DOI] [PubMed] [Google Scholar]
- 40.Modi SR, Collins JJ, Relman DA. Antibiotics and the gut microbiota. J Clin Invest. 2014;124:4212–4218. doi: 10.1172/JCI72333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, Patil KR, Bork P, Typas A. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bibbò S, Settanni CR, Porcari S, Bocchino E, Ianiro G, Cammarota G, Gasbarrini A. Fecal Microbiota Transplantation: Screening and Selection to Choose the Optimal Donor. J Clin Med. 2020;9 doi: 10.3390/jcm9061757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murtaza N, Burke LM, Vlahovich N, Charlesson B, O' Neill H, Ross ML, Campbell KL, Krause L, Morrison M. The Effects of Dietary Pattern during Intensified Training on Stool Microbiota of Elite Race Walkers. Nutrients. 2019;11 doi: 10.3390/nu11020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalton A, Mermier C, Zuhl M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes. 2019;10:555–568. doi: 10.1080/19490976.2018.1562268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Motiani KK, Collado MC, Eskelinen JJ, Virtanen KA, LÖyttyniemi E, Salminen S, Nuutila P, Kalliokoski KK, Hannukainen JC. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med Sci Sports Exerc. 2020;52:94–104. doi: 10.1249/MSS.0000000000002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation (FMT) an effective treatment for patients with functional gastrointestinal disorders (FGID)? Neurogastroenterol Motil. 2015;27:19–29. doi: 10.1111/nmo.12479. [DOI] [PubMed] [Google Scholar]
- 47.Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation the answer for irritable bowel syndrome? Am J Gastroenterol. 2014;109:1831–1832. doi: 10.1038/ajg.2014.295. [DOI] [PubMed] [Google Scholar]
- 48.Ostgaard H, Hausken T, Gundersen D, El-Salhy M. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol Med Rep. 2012;5:1382–1390. doi: 10.3892/mmr.2012.843. [DOI] [PubMed] [Google Scholar]
- 49.Bull MJ, Plummer NT. Part 2: Treatments for Chronic Gastrointestinal Disease and Gut Dysbiosis. Integr Med (Encinitas) 2015;14:25–33. [PMC free article] [PubMed] [Google Scholar]
- 50.Holvoet T, Joossens M, Wang J, Boelens J, Verhasselt B, Laukens D, van Vlierberghe H, Hindryckx P, De Vos M, De Looze D, Raes J. Assessment of faecal microbial transfer in irritable bowel syndrome with severe bloating. Gut. 2017;66:980–982. doi: 10.1136/gutjnl-2016-312513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chong CYL, Bloomfield FH, O'Sullivan JM. Factors Affecting Gastrointestinal Microbiome Development in Neonates. Nutrients. 2018;10 doi: 10.3390/nu10030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen QY, Tian HL, Yang B, Lin ZL, Zhao D, Ye C, Zhang XY, Qin HL, Li N. [Effect of intestinal preparation on the efficacy and safety of fecal microbiota transplantation treatment] Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:48–55. doi: 10.3760/cma.j.cn.441530-20200418-00225. [DOI] [PubMed] [Google Scholar]
- 53.Ianiro G, Maida M, Burisch J, Simonelli C, Hold G, Ventimiglia M, Gasbarrini A, Cammarota G. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: A systematic review and meta-analysis. United European Gastroenterol J. 2018;6:1232–1244. doi: 10.1177/2050640618780762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Salhy M, Hausken T, Hatlebakk JG. Increasing the Dose and/or Repeating Faecal Microbiota Transplantation (FMT) Increases the Response in Patients with Irritable Bowel Syndrome (IBS) Nutrients. 2019;11 doi: 10.3390/nu11061415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El-Salhy M, Valeur J, Hausken T, Gunnar Hatlebakk J. Changes in fecal short-chain fatty acids following fecal microbiota transplantation in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2021;33:e13983. doi: 10.1111/nmo.13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El-Salhy M, Casen C, Valeur J, Hausken T, Hatlebakk JG. Responses to faecal microbiota transplantation in female and male patients with irritable bowel syndrome. World J Gastroenterol. 2021;27:2219–2237. doi: 10.3748/wjg.v27.i18.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keller JJ, Vehreschild MJ, Hvas CL, Jørgensen SM, Kupciskas J, Link A, Mulder CJ, Goldenberg SD, Arasaradnam R, Sokol H, Gasbarrini A, Hoegenauer C, Terveer EM, Kuijper EJ, Arkkila P UEG working group of the Standards and Guidelines initiative Stool banking for FMT. Stool for fecal microbiota transplantation should be classified as a transplant product and not as a drug. United European Gastroenterol J. 2019;7:1408–1410. doi: 10.1177/2050640619887579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang S, Xu M, Wang W, Cao X, Piao M, Khan S, Yan F, Cao H, Wang B. Systematic Review: Adverse Events of Fecal Microbiota Transplantation. PLoS One. 2016;11:e0161174. doi: 10.1371/journal.pone.0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dailey FE, Turse EP, Daglilar E, Tahan V. The dirty aspects of fecal microbiota transplantation: a review of its adverse effects and complications. Curr Opin Pharmacol. 2019;49:29–33. doi: 10.1016/j.coph.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 60.DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, Turbett S, Chung RT, Chen YB, Hohmann EL. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl J Med. 2019;381:2043–2050. doi: 10.1056/NEJMoa1910437. [DOI] [PubMed] [Google Scholar]
- 61.Blaser MJ. Fecal Microbiota Transplantation for Dysbiosis - Predictable Risks. N Engl J Med. 2019;381:2064–2066. doi: 10.1056/NEJMe1913807. [DOI] [PubMed] [Google Scholar]
- 62.Barbara G, Ianiro G. Faecal microbial transplantation in IBS: ready for prime time? Gut. 2020;69:795–796. doi: 10.1136/gutjnl-2019-320411. [DOI] [PubMed] [Google Scholar]
- 63.Camilleri M. FMT in IBS: a call for caution. Gut. 2021;70:431. doi: 10.1136/gutjnl-2020-321529. [DOI] [PubMed] [Google Scholar]
- 64.Perler BK, Chen B, Phelps E, Allegretti JR, Fischer M, Ganapini V, Krajiceck E, Kumar V, Marcus J, Nativ L, Kelly CR. Long-Term Efficacy and Safety of Fecal Microbiota Transplantation for Treatment of Recurrent Clostridioides difficile Infection. J Clin Gastroenterol. 2020;54:701–706. doi: 10.1097/MCG.0000000000001281. [DOI] [PubMed] [Google Scholar]
- 65.Sun Q, Jia Q, Song L, Duan L. Alterations in fecal short-chain fatty acids in patients with irritable bowel syndrome: A systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e14513. doi: 10.1097/MD.0000000000014513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.El-Salhy M, Hatlebakk JG, Hausken T. Diet in Irritable Bowel Syndrome (IBS): Interaction with Gut Microbiota and Gut Hormones. Nutrients. 2019;11 doi: 10.3390/nu11081824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El-Salhy M. Irritable bowel syndrome: diagnosis and pathogenesis. World J Gastroenterol. 2012;18:5151–5163. doi: 10.3748/wjg.v18.i37.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.El-Salhy M, Hatlebakk JG, Gilja OH, Hausken T. Irritable bowel syndrome: recent developments in diagnosis, pathophysiology, and treatment. Expert Rev Gastroenterol Hepatol. 2014;8:435–443. doi: 10.1586/17474124.2014.888952. [DOI] [PubMed] [Google Scholar]
- 69.El-Salhy M, Seim I, Chopin L, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front Biosci (Elite Ed) 2012;4:2783–2800. doi: 10.2741/e583. [DOI] [PubMed] [Google Scholar]
- 70.El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, Hausken T. Is irritable bowel syndrome an organic disorder? World J Gastroenterol. 2014;20:384–400. doi: 10.3748/wjg.v20.i2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khan WI, Ghia JE. Gut hormones: emerging role in immune activation and inflammation. Clin Exp Immunol. 2010;161:19–27. doi: 10.1111/j.1365-2249.2010.04150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2013;20:14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 74.El-Salhy M, Solomon T, Hausken T, Gilja OH, Hatlebakk JG. Gastrointestinal neuroendocrine peptides/amines in inflammatory bowel disease. World J Gastroenterol. 2017;23:5068–5085. doi: 10.3748/wjg.v23.i28.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mawe GM, Coates MD, Moses PL. Review article: intestinal serotonin signalling in irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:1067–1076. doi: 10.1111/j.1365-2036.2006.02858.x. [DOI] [PubMed] [Google Scholar]
- 76.Erspamer V. Occurrence and distribution of 5-hydroxytryptamine (enteramine) in the living organism. Z Vitam Horm Fermentforsch. 1957;9:74–96. [PubMed] [Google Scholar]
- 77.Chen JX, Pan H, Rothman TP, Wade PR, Gershon MD. Guinea pig 5-HT transporter: cloning, expression, distribution, and function in intestinal sensory reception. Am J Physiol. 1998;275:G433–G448. doi: 10.1152/ajpgi.1998.275.3.G433. [DOI] [PubMed] [Google Scholar]
- 78.Keating C, Beyak M, Foley S, Singh G, Marsden C, Spiller R, Grundy D. Afferent hypersensitivity in a mouse model of post-inflammatory gut dysfunction: role of altered serotonin metabolism. J Physiol. 2008;586:4517–4530. doi: 10.1113/jphysiol.2008.156984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coleman NS, Foley S, Dunlop SP, Wheatcroft J, Blackshaw E, Perkins AC, Singh G, Marsden CA, Holmes GK, Spiller RC. Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin Gastroenterol Hepatol. 2006;4:874–881. doi: 10.1016/j.cgh.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 80.El-Salhy M, Gundersen D, Ostgaard H, Lomholt-Beck B, Hatlebakk JG, Hausken T. Low densities of serotonin and peptide YY cells in the colon of patients with irritable bowel syndrome. Dig Dis Sci. 2012;57:873–878. doi: 10.1007/s10620-011-1948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El-Salhy M, Patcharatrakul T, Hatlebakk JG, Hausken T, Gilja OH, Gonlachanvit S. Enteroendocrine, Musashi 1 and neurogenin 3 cells in the large intestine of Thai and Norwegian patients with irritable bowel syndrome. Scand J Gastroenterol. 2017;52:1331–1339. doi: 10.1080/00365521.2017.1371793. [DOI] [PubMed] [Google Scholar]
- 82.El-Salhy M, Wendelbo I, Gundersen D. Serotonin and serotonin transporter in the rectum of patients with irritable bowel disease. Mol Med Rep. 2013;8:451–455. doi: 10.3892/mmr.2013.1525. [DOI] [PubMed] [Google Scholar]
- 83.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mishima Y, Ishihara S. Molecular Mechanisms of Microbiota-Mediated Pathology in Irritable Bowel Syndrome. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21228664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mandić AD, Woting A, Jaenicke T, Sander A, Sabrowski W, Rolle-Kampcyk U, von Bergen M, Blaut M. Clostridium ramosum regulates enterochromaffin cell development and serotonin release. Sci Rep. 2019;9:1177. doi: 10.1038/s41598-018-38018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El-Salhy M, Grimelius L, Wilander E, Ryberg B, Terenius L, Lundberg JM, Tatemoto K. Immunocytochemical identification of polypeptide YY (PYY) cells in the human gastrointestinal tract. Histochemistry. 1983;77:15–23. doi: 10.1007/BF00496632. [DOI] [PubMed] [Google Scholar]
- 87.El-Salhy M, Hatlebakk JG, Hausken T. Possible role of peptide YY (PYY) in the pathophysiology of irritable bowel syndrome (IBS) Neuropeptides. 2020;79:101973. doi: 10.1016/j.npep.2019.101973. [DOI] [PubMed] [Google Scholar]
- 88.Cox HM, Pollock EL, Tough IR, Herzog H. Multiple Y receptors mediate pancreatic polypeptide responses in mouse colon mucosa. Peptides. 2001;22:445–452. doi: 10.1016/s0196-9781(01)00355-2. [DOI] [PubMed] [Google Scholar]
- 89.Cox HM, Tough IR. Neuropeptide Y, Y1, Y2 and Y4 receptors mediate Y agonist responses in isolated human colon mucosa. Br J Pharmacol. 2002;135:1505–1512. doi: 10.1038/sj.bjp.0704604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sheikh SP, Williams JA. Structural characterization of Y1 and Y2 receptors for neuropeptide Y and peptide YY by affinity cross-linking. J Biol Chem. 1990;265:8304–8310. [PubMed] [Google Scholar]
- 91.Inui A, Sano K, Miura M, Hirosue Y, Nakajima M, Okita M, Baba S, Kasuga M. Evidence for further heterogeneity of the receptors for neuropeptide-Y and peptide-YY in tumor cell lines derived from neural crest. Endocrinology. 1992;131:2090–2096. doi: 10.1210/endo.131.5.1330489. [DOI] [PubMed] [Google Scholar]
- 92.Mao YK, Wang YF, Ward G, Cipris S, Daniel EE, McDonald TJ. Peptide YY receptor in submucosal and myenteric plexus synaptosomes of canine small intestine. Am J Physiol. 1996;271:G36–G41. doi: 10.1152/ajpgi.1996.271.1.G36. [DOI] [PubMed] [Google Scholar]
- 93.Walsh DA, Wharton J, Blake DR, Polak JM. Localization and characterization of neuropeptide Y binding sites in porcine and human colon. Br J Pharmacol. 1993;108:304–311. doi: 10.1111/j.1476-5381.1993.tb12800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wharton J, Gordon L, Byrne J, Herzog H, Selbie LA, Moore K, Sullivan MH, Elder MG, Moscoso G, Taylor KM. Expression of the human neuropeptide tyrosine Y1 receptor. Proc Natl Acad Sci USA. 1993;90:687–691. doi: 10.1073/pnas.90.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gregor P, Feng Y, DeCarr LB, Cornfield LJ, McCaleb ML. Molecular characterization of a second mouse pancreatic polypeptide receptor and its inactivated human homologue. J Biol Chem. 1996;271:27776–27781. doi: 10.1074/jbc.271.44.27776. [DOI] [PubMed] [Google Scholar]
- 96.Gregor P, Millham ML, Feng Y, DeCarr LB, McCaleb ML, Cornfield LJ. Cloning and characterization of a novel receptor to pancreatic polypeptide, a member of the neuropeptide Y receptor family. FEBS Lett. 1996;381:58–62. doi: 10.1016/0014-5793(96)00067-1. [DOI] [PubMed] [Google Scholar]
- 97.Yan H, Yang J, Marasco J, Yamaguchi K, Brenner S, Collins F, Karbon W. Cloning and functional expression of cDNAs encoding human and rat pancreatic polypeptide receptors. Proc Natl Acad Sci USA. 1996;93:4661–4665. doi: 10.1073/pnas.93.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gué M, Junien JL, Reeve JR Jr, Rivier J, Grandt D, Taché Y. Reversal by NPY, PYY and 3-36 molecular forms of NPY and PYY of intracisternal CRF-induced inhibition of gastric acid secretion in rats. Br J Pharmacol. 1996;118:237–242. doi: 10.1111/j.1476-5381.1996.tb15393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hyland NP, Cox HM. The regulation of veratridine-stimulated electrogenic ion transport in mouse colon by neuropeptide Y (NPY), Y1 and Y2 receptors. Br J Pharmacol. 2005;146:712–722. doi: 10.1038/sj.bjp.0706368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hyland NP, Sjöberg F, Tough IR, Herzog H, Cox HM. Functional consequences of neuropeptide Y Y 2 receptor knockout and Y2 antagonism in mouse and human colonic tissues. Br J Pharmacol. 2003;139:863–871. doi: 10.1038/sj.bjp.0705298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hamer HM, Jonkers DM, Bast A, Vanhoutvin SA, Fischer MA, Kodde A, Troost FJ, Venema K, Brummer RJ. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr. 2009;28:88–93. doi: 10.1016/j.clnu.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 102.Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, Neunlist M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1772–1782. doi: 10.1053/j.gastro.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 103.Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, Danna SC, Tripathy S, Hegsted M, Keenan MJ. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. 2008;295:E1160–E1166. doi: 10.1152/ajpendo.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324:353–360. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- 105.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 106.Zhang J, Song L, Wang Y, Liu C, Zhang L, Zhu S, Liu S, Duan L. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J Gastroenterol Hepatol. 2019;34:1368–1376. doi: 10.1111/jgh.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Long X, Li M, Li LX, Sun YY, Zhang WX, Zhao DY, Li YQ. Butyrate promotes visceral hypersensitivity in an IBS-like model via enteric glial cell-derived nerve growth factor. Neurogastroenterol Motil. 2018;30:e13227. doi: 10.1111/nmo.13227. [DOI] [PubMed] [Google Scholar]
- 108.Banasiewicz T, Krokowicz Ł, Stojcev Z, Kaczmarek BF, Kaczmarek E, Maik J, Marciniak R, Krokowicz P, Walkowiak J, Drews M. Microencapsulated sodium butyrate reduces the frequency of abdominal pain in patients with irritable bowel syndrome. Colorectal Dis. 2013;15:204–209. doi: 10.1111/j.1463-1318.2012.03152.x. [DOI] [PubMed] [Google Scholar]
- 109.Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 110.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512–519, e114. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 111.Valeur J, Røseth AG, Knudsen T, Malmstrøm GH, Fiennes JT, Midtvedt T, Berstad A. Fecal Fermentation in Irritable Bowel Syndrome: Influence of Dietary Restriction of Fermentable Oligosaccharides, Disaccharides, Monosaccharides and Polyols. Digestion . 2016;94:50–56. doi: 10.1159/000448280. [DOI] [PubMed] [Google Scholar]