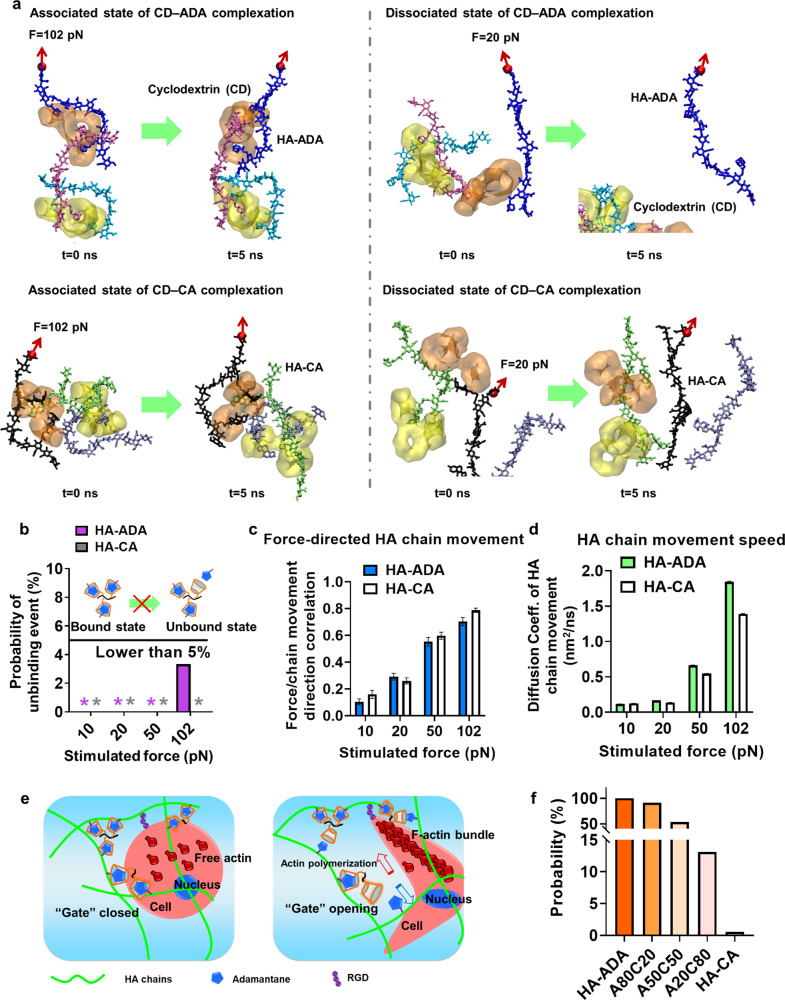
Fig. 3. MD and KMC simulation results verify the key role of binding kinetics of host–guest crosslinks in enabling cell-mediated hydrogel network reorganization.
a MD simulation snapshots showing an application of a force on one end (red dot) of the HA segment when the host–guest crosslink in HA–ADA or HA–CA hydrogels is in the bound and the unbound state, respectively. Two oligomerized acryloyl β-cyclodextrin crosslinkers (each contains three CDs) are shown in orange and yellow, respectively. b For a given magnitude of applied force, sixty 5-ns simulations were performed with CD–ADA pairs in the bound state. Among the total 240 simulations, unbinding events were only observed in two simulations with F = 102 pN. * means that the value is 0. For the CD–CA pairs in the bound state, 60 simulations performed at F = 102 pN revealed no unbinding event. c In a separate set of 480 5-ns simulations with a given CD–ADA or CD–CA pair initially in the unbound state, we measured the correlation coefficient (value ranges from −1 to 1) between the direction of the applied force and the movement of the corresponding HA chain. Error bars represent the standard error of the mean. n = 60. d In the same set of simulations shown in c, the “speed” of HA chain movement is characterized by the diffusion coefficient of the guest molecule closest to the HA end being pulled. The sixty simulations performed at a given force are combined to estimate the diffusion coefficient from a linear regression model, the error of which is smaller than the line width (Supplementary Table 3). e Schematics of parallel actin bundles in filopodia and the crosslinked HA network they face. f Probability for a gate-opening event to occur within the estimated timescale of actin polymerization (~0.01 s) at F = 10 pN based on sets of 100,000 KMC calculations assuming n = 2 in acrylated host complexes (see Supplementary Information for details).