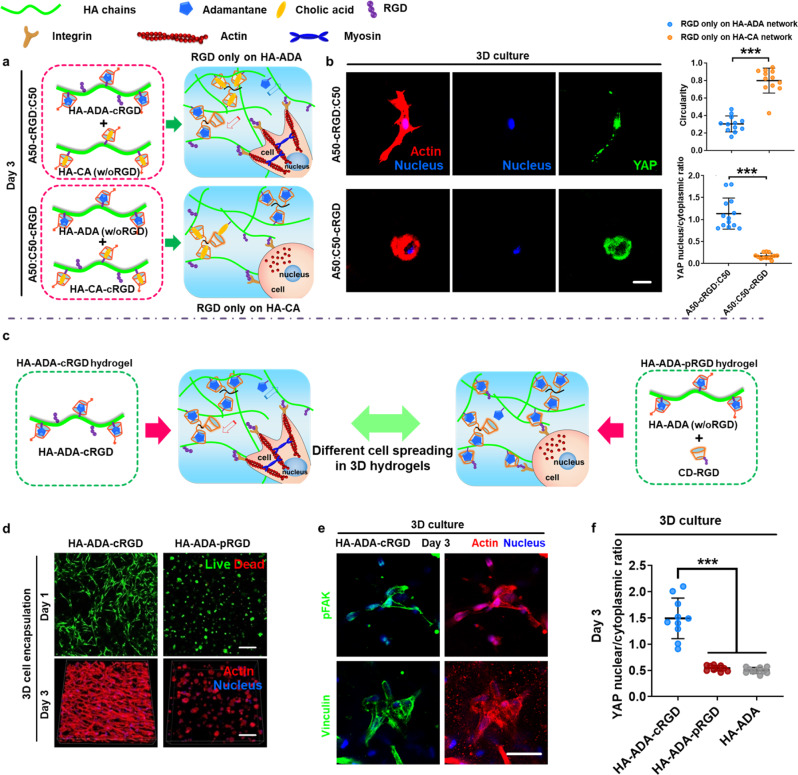
Fig. 4. The precise and covalent conjugation of cell-adhesive ligands to the hydrogel subnetwork connected by crosslinks with fast binding kinetics is required for efficient 3D spreading and mechanosensing of hMSCs.
a Schematic illustration of the preparation of A50C50 hydrogels with selective RGD conjugations and 3D cell culture in the hydrogels. RGD peptide was only conjugated either to the HA–ADA subnetwork (A50-cRGD:C50) or to HA–CA subnetwork (A50:C50-cRGD) in the A50C50 hydrogels. b Representative immunofluorescence staining against F-actin (red), nuclei (blue), and YAP (green) in hMSCs encapsulated in A50C50 hydrogels with selective RGD conjugations after 3 days of culture (scale bar = 25 μm) and quantification of the circularity and nuclear YAP fluorescence intensity (intensity ratio between the nucleus and cytoplasm). Data are presented as mean values ± SD, n = 12 cells per group from two independent hydrogels; ***p < 0.001 (two-tailed Student’s t-test). c Schematic illustration of the preparation of hydrogels with different RGD conjugation methods (HA–ADA–cRGD and HA–ADA–pRGD) and 3D cell culture with different RGD conjugation methods. d Representative images of hMSCs encapsulated within hydrogels (3D cell encapsulation) with different RGD conjugation methods (HA–ADA–cRGD and HA–ADA–pRGD) after 1 and 3 days of culture. Scale bar = 200 µm. e Representative immunofluorescence staining against F-actin (red), nuclei (blue) and pFAK or vinculin (green) in hMSCs cultured in highly dynamic HA–ADA–cRGD hydrogels for 3 days. Scale bar = 50 μm. f Quantification of the nuclear YAP fluorescence intensity (intensity ratio between the nucleus and cytoplasm) in hMSCs encapsulated in hydrogels with different RGD conjugation methods (HA–ADA–cRGD, HA–ADA–pRGD, and HA–ADA) on after 3 days of culture. Data are presented as mean values ± SD, n = 10 cells per group from two independent hydrogels; ***p < 0.001 (two-tailed Student’s t-test).