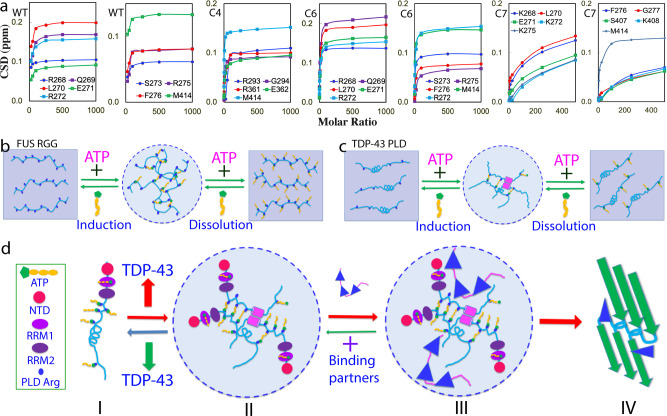
Fig. 7. Speculative model for ATP to modulate LLPS of cytoplasmic TDP-43.
a Concentration-dependent CSD for the significantly perturbed residues of TDP-43 WT-, C4-, C6-, and C7-PLD. b The speculative mechanism for ATP to biphasically modulate LLPS of RGG-rich FUS CTD. c The speculative mechanism for ATP to biphasically modulate LLPS of TDP-43 PLD. d A speculative model for ATP to modulate LLPS of cytoplasmic TDP-43 and its pathological implications. (I) Under the physiological conditions, LLPS is inhibited due to being bound with ATP of cytoplasmic TDP-43 in neurons, where TDP-43 has concentrations of ~1 μM while ATP has concentrations of ~3 mM. (II) Cytoplasmic TDP-43 reversibly phase separates into dynamic droplets which might be enhanced by the bivalent binding of ATP under pathological/aging conditions with the accumulation of TDP-43, or/and reduction of ATP concentrations. (III) Cytoplasmic TDP-43 droplets become capable of recruiting other proteins. (IV) With a long incubation time, TDP-43 droplets may undergo an irreversible exaggeration from the dynamic droplets into aggregates or/and amyloid fibrils characteristic of ALS.