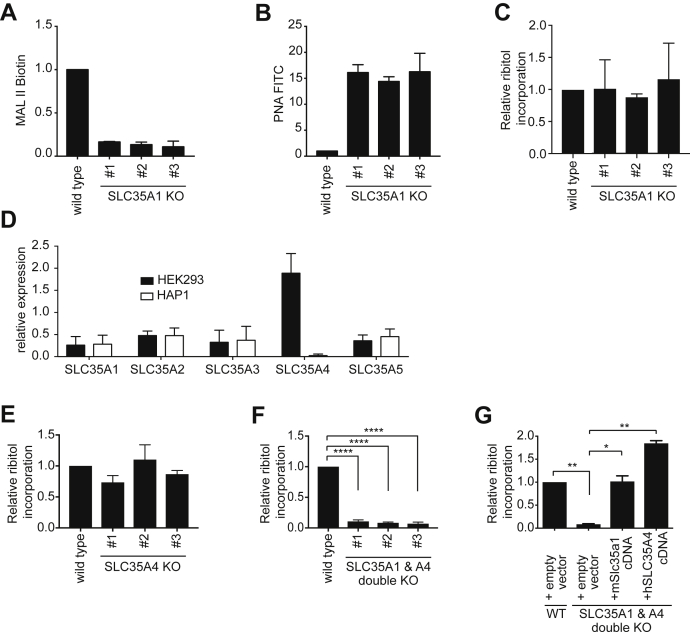
Figure 2.
SLC35A1 and SLC35A4 play redundant role in CDP-ribitol transport in HEK293 cells.A and B, wildtype HEK293 or three different SLC35A1 KO HEK293 cell lines were analyzed by flow cytometry with MAL II (A) and PNA lectin (B). Means ± SD of the mode of fluorescence intensity of two independent experiments are shown. C, ribitol incorporation into matriglycan was assessed by GC/MS on α-dystroglycan (aa 1–485) affinity purified from wildtype and three different SLC35A1 KO HEK293 clones. Means ± SD of three independent experiments are shown. D, RT-quantitative PCR of the indicated transcripts in HEK293 and HAP1 cells. Values were normalized to the mean of b2-microglobulin (B2M) and TATA-binding protein (TBP) and are presented as means ± SD of three samples. E–G, ribitol incorporation was determined as described for C, but in SLC35A4 KO HEK293 clones (E), SLC35A1 and SLC35A4 double KO HEK293 clones (F), as well as double KO HEK293 clones where expression of either mSlc35a1 or SLC35A4 was restored by lentiviral transduction (G). Empty vector indicates transduction with the empty lentiviral backbone. Values are means ± SD of three independent experiments. HEK293, human embryonic kidney 293 cells; MAL II, Maackia amurensis lectin II; PNA, peanut agglutinin.