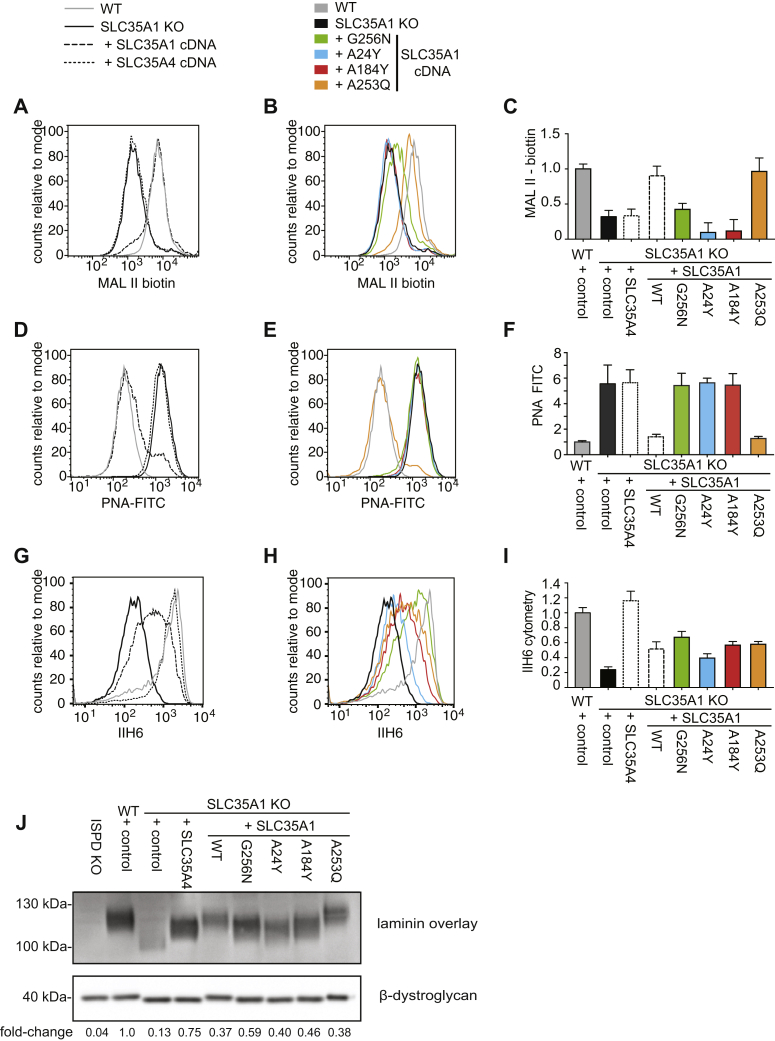
Figure 6.
Restricting the catalytic site of SLC35A1 abolishes CMP-sialic acid but still allows CDP-ribitol transport.A–I, HAP1 wildtype or SLC35A1 KO cells engineered to express SLC35A1 or the indicated SLC35A1 mutants were analyzed by flow cytometry with MAL II lectin, PNA, and IIH6 antibody (A and B, D and E, and G and H). Flow cytometry data were divided into two panels for the sake of clarity. Quantification of three independent experiments is shown in panels C, F, and I. Means ± SEM or mean fluorescence intensities are shown. J, laminin overlay and β-dystroglycan Western blot were performed in the same cell lines as described previously in addition to ISPD KO cells. Quantification of the relative signal obtained in laminin overlay was normalized to the β-dystroglycan Western blot signal. ISPD, isoprenoid synthase domain–containing protein; MAL II, Maackia amurensis lectin II; PNA, peanut agglutinin.