Dear Editor,
As patients with cancer constitute a vulnerable population at higher risk of contracting severe COVID-19, they represent a priority group for SARS-CoV-2 vaccination. However, despite favouring these patients theoretically, patients with cancer were excluded from major registration trials on SARS-CoV-2 vaccines [1]. Furthermore, two recent reports on the immunogenicity of mRNA vaccines in patients with cancer excluded those from the study having previous SARS-CoV-2 exposure [2,3]. We compared IgG antibody levels after one or two doses of BNT162b2 (Pfizer-BioNTech) vaccine in patients with cancer with or without prior SARS-CoV-2 infection.
All patients with an reverse transcription polymerase chain reaction (RT-PCR) confirmed (N = 74) or serologically detected (N = 15) SARS-CoV-2 infection (CoV-positive) who underwent vaccination with BNT162b2 (Pfizer-BioNTech) between 05/03/2021 and 07/05/2021 at the outpatient department of the ‘Franz Tappeiner’ Hospital Merano were included in this retrospective study. In addition, a control group including 154 consecutive patients with cancer without documented preexisting SARS-CoV-2 immunity (CoV-negative) was analysed. After a median time of 24 days (range: 10–68) after infection, CoV -positive patients underwent baseline blood testing for detection of IgG antibodies against the nucleocapsid (N)-protein and to the receptor-binding domain of the S1 subunit of the spike (S)-protein of SARS-CoV-2. In CoV-negative patients N/S-protein was measured on the same day of administration of the first vaccine dose. At week three after the first vaccine dose, all patients repeated blood tests to quantitatively assess S-IgG antibody levels. Serum anti-N/S IgG were detected using the Abbot SARS-CoV-2 IgG chemiluminescent microparticle immunoassay, according to the manufacturer's instructions. The results were expressed as arbitrary units (AU) per millilitre, and S-IgG values of ≥50 AU were interpreted as seropositive (upper limit: 40,000 AU/mL). CoV -positive patients who were still seronegative for S-IgG after the first vaccine dose received a second dose, whereas all CoV-negative patients were fully vaccinated with two doses. The study was approved by the local Ethics Committee of the ‘Südtiroler Sanitätsbetrieb’amended protocol number 35/2020.
Demographic and clinical characteristics of the patients are summarised in the Supplementary Table 1. At baseline, 55 of 89 (61.8%) CoV -positive patients showed positive S-IgG antibodies, whereas 19 of 89 (21.3%) were S-IgG negative. In 15/89 (16.9%) cases S-IgG was not available as prior SARS-CoV-2 infection was detected serologically shortly before vaccination (all seropositive for N-protein IgG). After 1 vaccine dose, seropositive CoV-positive patients increased to 81 of 89 (91.0%; S-IgG median value 15,927 AU/mL, range: 0–40000) cases, and to 86/89 (96.6%) after the second dose, respectively (Supplementary Fig. 1). In contrast, seroconversion in CoV-negative patients who were all negative for N/S-IgG at baseline was demonstrated in 94 of 154 (61.0%; median S-IgG value 101.2 AU/mL, range: 0–38,727) cases after the first vaccine dose, and in 132 of 154 (85.7%) after the second dose, respectively. In median, the S-IgG antibody titres in CoV -positive patients were 157.3-fold higher than that of CoV -negative patients after the first vaccine dose (p < 0.001, Mann-Whitney-U test, Fig. 1 ).
Fig. 1.
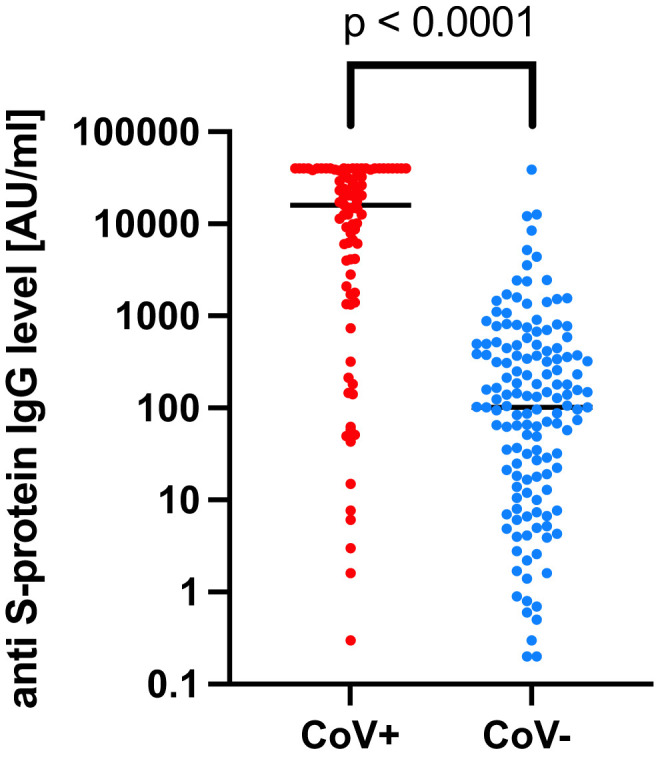
Quantitative SARS-CoV-2 spike IgG antibody levels in patients with (CoV-positive; N = 81) and without (CoV-negative; N = 94) prior SARS-CoV-2 infection after one dose of BNT162b2 (Pfizer/BioNtech) mRNA vaccine. Median titres (horizontal lines) of anti-S IgG were compared between CoV-positive and CoV-negative patients using a Mann-Whitney-U test.
Our results demonstrate that a single dose of mRNA vaccine induce high level of S-IgG in the majority of patients with cancer with prior SARS-CoV-2 infection. These data are in line with two recent reports among nursing home residents and healthcare workers previously infected with SARS-CoV-2 [4,5]. However, immunogenicity in cancer patients without previous infection was lower after one dose but significantly increased within 3 weeks of a BNT162b2 vaccine boost at day 21 after the first dose. Screening for S-IgG antibodies may be helpful to further optimise vaccine doses in individuals with or without prior SARS-CoV-2 infection. The duration of antibody response and longitudinal effectiveness of mRNA vaccines in patients with cancer need further investigation.
Disclosures
No disclosures were reported.
Ethical approval
The study was approved by the local ethics committee of the ‘Südtiroler Sanitätsbetrieb’ (amended protocol number 35–2020) and was performed in accordance with national regulations and the Declaration of Helsinki and all its amendments.
Funding
This work has been supported by the budget of the Südtiroler Sanitätsbetrieb.
Data availability
The data sets used and analysed during the present study are available from the corresponding author on reasonable request.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2021.05.036.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Multimedia component 1
Positive rate of IgG spike antibodies against SARS-CoV-2 in patients with (CoV-positive) and without (CoV-negative) prior SARS-CoV-2 infection after one and two doses of BNT162b2 (Pfizer/BioNtech) mRNA vaccine. P values as determined by Fisher's exact test.
References
- 1.Tougeron D., Hentzien M., Seitz-Polski B., Bani-Sadr F., Bourhis J., Ducreux M., et al. for Thésaurus National de Cancérologie Digestive (TNCD) réseau de Groupes Coopérateurs en Oncologie (GCO) Fédération Nationale des Centres de Lutte Contre le Cancer (UNICANCER) Association de Chirurgie Hépato-Bilio-Pancréatique et Transplantation (ACHBT) Association de Recherche sur les Cancers Gynécologiques-Groupes d'Investigateurs Nationaux pour l'étude des Cancers Ovariens et du Sein (ARCAGY-GINECO) Fédération Francophone de Cancérologie Digestive (FFCD) Groupe Coopérateur multidisciplinaire en Oncologie (GERCOR) Groupe d'Oncologie Radiothérapie Tête et Cou-Intergroupe ORL (GORTEC-Intergroupe ORL) Intergroupe Francophone de Cancérologie Thoracique (IFCT) InterGroupe Coopérateur de Neuro-Oncologie/Association des Neuro-Oncologues d'Expression Française (IGCNO-ANOCEF) Société Française de Chirurgie Digestive (SFCD) Société Française d'Endoscopie Digestive (SFED) Société Française de Radiothérapie Oncologique (SFRO) Société Française de Radiologie (SFR) Société Nationale Française de Colo-Proctologie (SNFCP) Société Nationale Française de Gastroentérologie (SNFGE) Severe acute respiratory syndrome coronavirus 2 vaccination for patients with solid cancer: review and point of view of a French oncology intergroup (GCO, TNCD, UNICANCER) Eur J Cancer. 2021;150:232–239. doi: 10.1016/j.ejca.2021.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jérôme B., Emmanuel C., Zoubir A., Olivier C., Abakar M., Sabine M., et al. Impaired immunogenicity of BNT162b2 anti SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol. 2021;S0923–7534(21) doi: 10.1016/j.annonc.2021.04.019. 01183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monin L., Laing A.G., Muñoz-Ruiz M., McKenzie D.R., Del Molino Del Barrio I., Alaguthurai T., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;S1470–2045(21):213–218. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blain H., Tuaillon E., Gamon L., Pisoni A., Miot S., Picot M.C., et al. Spike antibody levels of nursing home residents with or without prior COVID-19 3 Weeks after a single BNT162b2 vaccine dose. J Am Med Assoc. 2021 Apr 15;325(18):1898–1899. doi: 10.1001/jama.2021.6042. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saadat S., Rikhtegaran Tehrani Z., Logue J., Newman M., Frieman M.B., Harris A.D., et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. J Am Med Assoc. 2021 Apr 13;325(14):1467–1469. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1
Positive rate of IgG spike antibodies against SARS-CoV-2 in patients with (CoV-positive) and without (CoV-negative) prior SARS-CoV-2 infection after one and two doses of BNT162b2 (Pfizer/BioNtech) mRNA vaccine. P values as determined by Fisher's exact test.
Data Availability Statement
The data sets used and analysed during the present study are available from the corresponding author on reasonable request.


