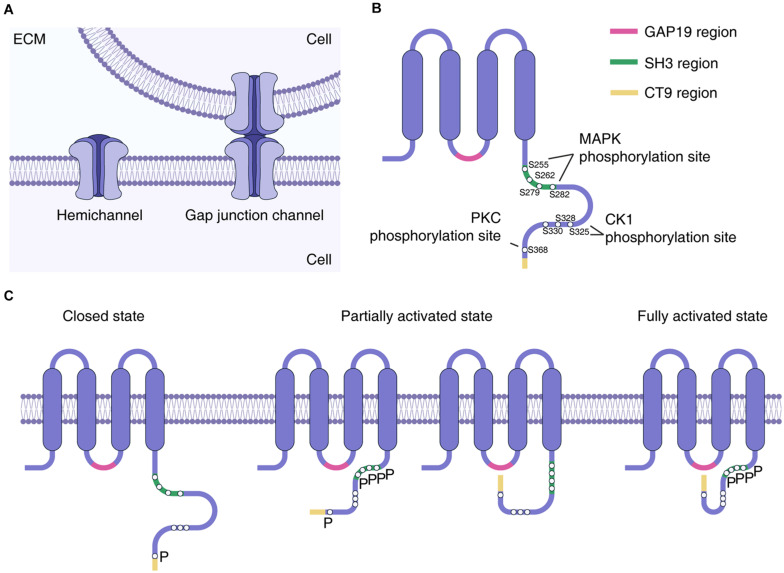
FIGURE 1.
Connexin formation of hemichannel. (A) Connexin hexamer constitutes hemichannel, while hemichannels in the adjacent cells interact to form the gap junction channel. (B) Structure of Cx43 protein. Phosphorylation sites by MAPK, CK1, and PKC in the c-terminal tail are highlighted by white circles. Regions crucial for hemichannel activation regulation was also highlighted. (C) Proposed conformation changes that lead to hemichannel activation. Interaction of either CT9 or SH3-binding region with GAP19 region could achieve partial hemichannel activation, while interaction of both CT9 and SH3-binding region with GAP19 lead to fully activation of hemichannel (Iyyathurai et al., 2018). MAPK phosphorylation at S255, S262, S279, and S282 sites was proposed to facilitate interaction of SH3-binding region to the GAP19 region, enabling hemichannel activation (Freitas-Andrade et al., 2019). PKC phosphorylation at S386 could reduce the permeability of larger molecules such as sucrose (Bao et al., 2007; Hawat and Baroudi, 2008), which might act to interfere with the interaction between CT9 and GAP19 region. CK1 phosphorylation at S325, S328, and S330 has been shown to modulate hemichannel activity (Ek-Vitorín et al., 2018), but the mechanism is yet to be determined. ECM, extracellular matrix; MAPK, mitogen activated protein kinase; PKC, protein kinase C; CK1, casein kinase 1; SH3, SRC Homology 3; CT9, last 9 amino acids of the Cx43 C terminus; P labels phosphorylated amino acid residue.