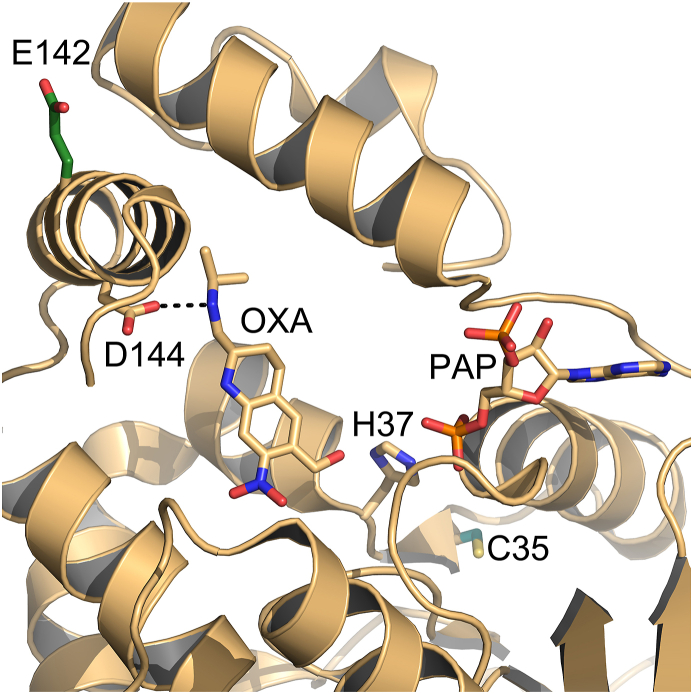
Fig. 5.
Crystal structure of SmSULT with highlighted resistance mutations. E142del, shown in dark green, is predicted to disrupt an α helix that contains D144, which makes a specific interaction with oxamniquine. The C35R mutation (teal) likely disrupts co-substrate PAPS binding as it is predicted to displace secondary structures and nearby H37 (proposed to stabilize the transition state). Some secondary structure has been removed for clarity. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)