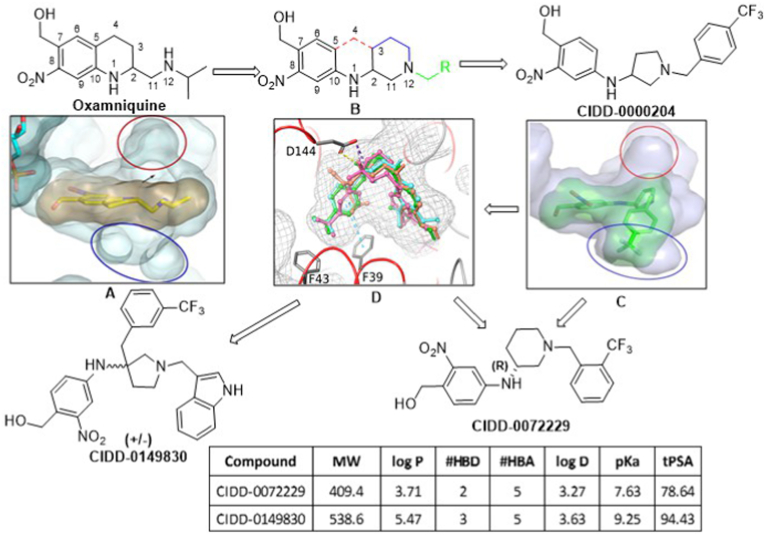
Fig. 7.
New compound design. The design strategy involved modifying oxamniquine by removing the element of rigidity by breaking the tetrahydroquinoline ring system shown in structure B (red bonds), introduce two rotatable bonds between C10–N1 and N1–C2, new substituted cyclic systems between C2 to N12 (structure B, blue bonds) and various R groups (Structure B, green) to capitalize on SmSULT, ShSULT and SjSULT residue interactions. We retained the critical ortho-nitro benzyl alcohol moiety necessary for activation by SULT. The new design allows for more simplistic chemistry as compared to OXA structure. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)