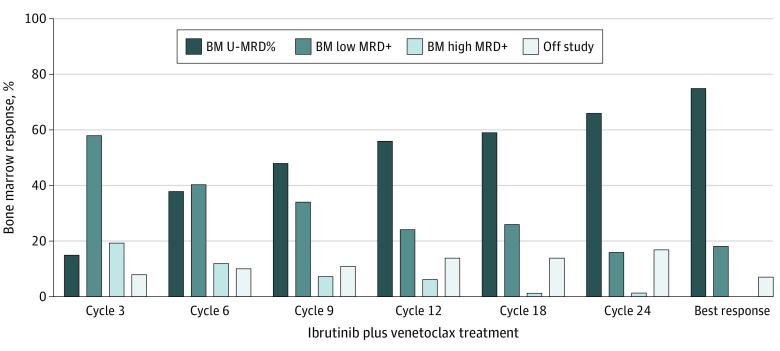
Figure 1. Bone Marrow (BM) Measurable Residual Disease (MRD) Response at Serial Points on an Intent-to-Treat Basis in 80 Patients.
Bone marrow MRD responses at serial points of combined ibrutinib and venetoclax therapy are shown; best response at any time during the study period is shown. The 5 patients who discontinued the study during ibrutinib monotherapy and never started venetoclax were included in the analysis. MRD was assessed by flow cytometry in bone marrow with a sensitivity of 10−4. Undetectable MRD (U-MRD), less than 0.01%; low MRD-positive (MRD+), 0.01% to less than 1%; and high MRD-positive, greater than or equal to 1%.