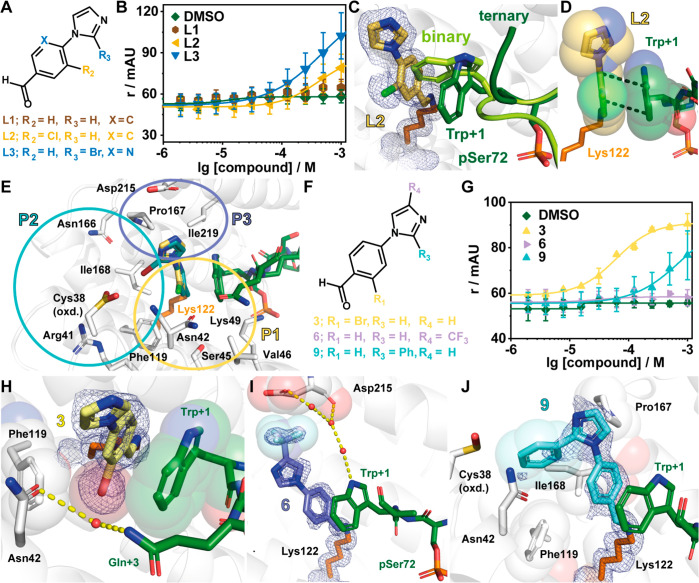
Figure 2.
Imine-based tethering revealed L2 and L3 as promising starting points for the development of Pin1/14-3-3 stabilizers. (A) Chemical structures of L1, L2, and L3. (B) Fluorescence anisotropy (r in mAU) assay of fragments L1–L3 where the compound was titrated to 10 μM 14-3-3γ and 50 nM fluorescently labeled Pin1. Shown are the mean ± SD (n = 3). (C) Ternary structure of 14-3-3/Pin1_72/L2 complex (PDB code 7AXN) overlaid with the binary structure of 14-3-3/Pin1_72 (PDB code 7AOG). Shown is the rearrangement of Trp+1 of Pin1 (binary, light green; ternary, dark green) upon binding of L2 (yellow sticks). 2Fo – Fc electron density map (blue mesh) is contoured at 1σ. (D) The benzaldehyde core of L2 (yellow sticks) forms π–π stacks with the indole moiety of Trp+1 of Pin1 (green sticks). (E) Overlay of L2 and L3 showing three pockets that can be probed during fragment optimization (PDB codes 7AXN and 7AYF). (F) Chemical structures of 3, 6, and 9. (G) Compounds were titrated to 10 μM 14-3-3γ and 50 nM Pin1_72 in FA (r, mAU). Shown are the mean ± SD (n = 3). (H–J) Crystal structures of 3 (PDB code 7NIG), 6 (PDB code 7NJ6), and 9 (PDB code 7NJA) bound to 14-3-3σ in complex with Pin1_72. Shown are hydrogen bonds (yellow dashes) and potential hydrophobic contacts (indicated by sphere representation) between 3 (H), 6 (I), and 9 (J) and 14-3-3σΔC (white cartoon and sticks). The 2Fo – Fc electron density map (blue mesh) is contoured at 1σ.