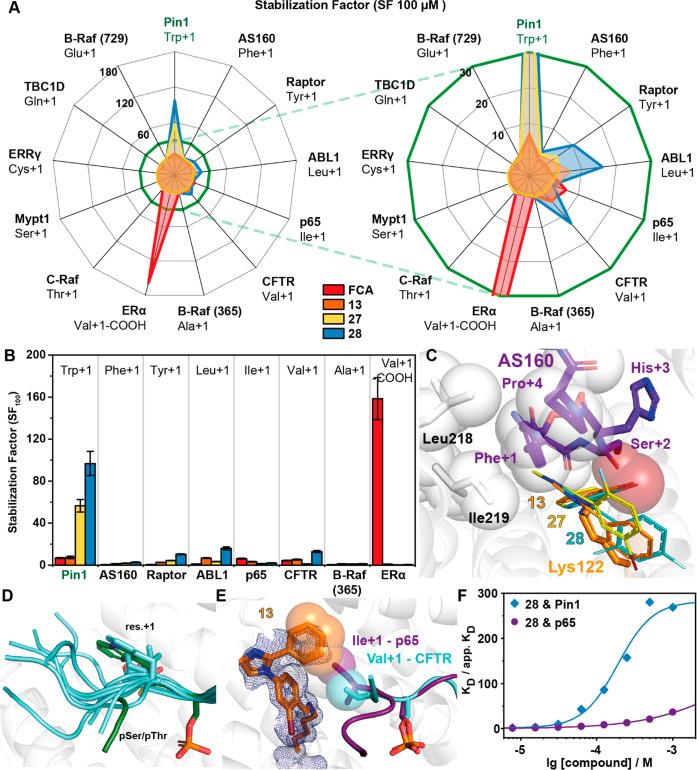
Figure 4.
Investigation of 13 representative 14-3-3/peptide interactions reveals selective stabilization of the 14-3-3/Pin1_72 complex by 28. (A) Radar plot of the SFs determined by FA protein titrations in the presence of 100 μM fragment. Fragment 28 shows preferential binding for the Pin1_72/14-3-3γ comparable to the effect of FCA on the ERα/14-3-3γ interaction. Right: close-up. (B) SF values determined with 14-3-3γ titrations in the presence of 100 μM 13, 27, or 28 in FA assays (n = 2). (C) Overlay of the binding pose of 13, 27, and 28 (line representation) with the AS160 binding epitope (violet sticks; PDB code 7NIX). (D) Structural overlay of the known 14-3-3 binding epitopes used in this study. (E) Overlay of crystal structures of 13 (orange sticks, 2Fo – Fc map at 1σ as blue mesh) binding to the p65_45 (violet sticks, carton)/14-3-3γ complex (PDB code 7NQP) and the CFTR (cyan sticks, cartoon)/14-3-3 complex (PDB code 5D3F, FC-A hidden for clarity). Hydrophobic contacts between 13 and Ile+1 of p65 and Val+1 of CFTR are indicated by transparent spheres. (F) Cooperative analysis of ternary complex formation using 28 with Pin1 and p65 peptides shows that stabilization of the ternary complex is driven by the unique environment created by the partner peptide binding.