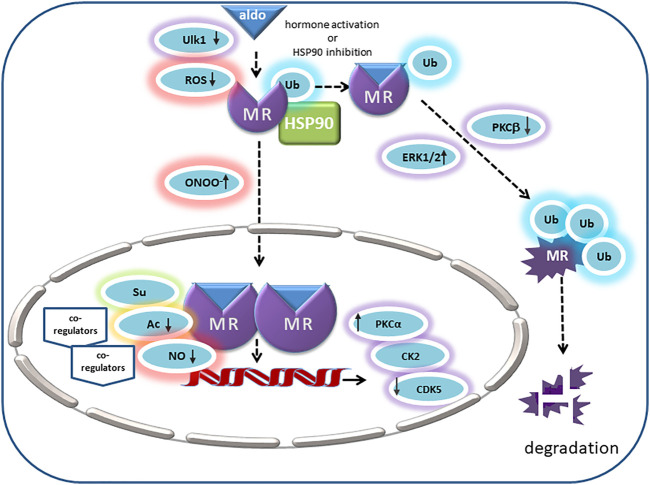
FIGURE 2.
In its unliganded state the MR is monoubiquitinated and located in the cytosol with chaperone molecules like HSP90. Its endogenous ligand aldosterone binds to MR in the cytosol and thereby triggers nuclear translocation. Ligand binding is inhibited by MR oxidation. In intercalated cells, autophagy activating kinase 1 (Ulk1) phosphorylates MR S843 in the LBD, mitigates its affinity for agonists and reducing its transcriptional activity. Peroxynitrite (ONOO-) can cause ligand-independent nuclear translocation and activation of MR. Inhibition of HSP90 or activation by aldosterone causes polyubiquitination of MR and degradation. ERK1/2 and PKC also modulate MR polyubiquitination and degradation. Positive regulators of MR transactivation activity are PKCa and CK2. In the prescence of cytokines, CK2 inhibits GRE signaling but promotes NFKB signaling and transcription of pro-inflammatory cytokines. CDK5 inhibits MR transactivation in neuronal cells. Acetylation (Ac) and SUMOylation (Su) lead to modulation of aldosterone-activated MR transactivation by direct and indirect effects. NO attenuates binding of MR to DNA, consequently reducing the MR’s transcriptional activity (↑ = stimulatory effect; ↓ = inhibitory effect).