Abstract
Neuroendocrine tumors (NETs) consist of a relatively rare spectrum of malignancies that can arise from neuroendocrine cells; lung NETs (L-NETs) represent about 25% of primary lung neoplasm and 10% of all carcinoid tumors. Diagnostic algorithm usually takes into consideration chest X-ray, contrast-enhanced CT and MRI. Nuclear medicine plays a crucial role in the detection and correct assessment of neoplastic functional status as it provides in vivo metabolic data related to the over-expression of Somatostatin Receptors (SSTRs) and also predicting response to peptide receptor radionuclide therapy (PRRT). 111In-Pentreotide (Octreoscan®) is commercially available for imaging of neuroendocrine tumors, their metastases and the management of patients with NETs. More recently, 99mTc-EDDA/HYNIC-TOC(Tektrotyd®) was introduced into the market and its use has been approved for imaging of patients with L-NETs and other SSTR-positive tumors. 99mTc-EDDA/HYNIC-TOC could also represent a good alternative to 68Ga-DOTA-peptides (DOTA-TOC, DOTA-NOC, DOTA-TATE) in hospitals or centers where PET/CT or 68Ge/68Ga generators are not available. When compared to 111In-Pentetreotide, Tektrotyd® showed slightly higher sensitivity, in the presence of higher imaging quality and lower radiation exposure for patients. Interesting perspectives depending on the kinetic analysis allowed by Tektrotyd® may be obtained in differential diagnosis of non-small cells lung cancer (NSCLC) versus small cells lung cancer (SCLC) and NETs. An interesting perspective could be also associated with a surgery radio-guided by Tektrotyd® in operable lung tumors, including either NETs and NSCLC.
Keywords: Neuroendocrine tumors, lung, nuclear medicine, PET/CT, diagnostic imaging, somatostatin, 99mTc-EDDA/HYNIC-TOC
1. Introduction
Neuroendocrine tumors (NETs) consist of a relatively rare spectrum of malignancies that can arise from neuroendocrine cells throughout the body, but are mainly found in the gastroenteropancreatic and bronchopulmonary systems [1]. During the last decades, NETs witnessed an increase in both incidence and prevalence mainly due to improved sensibility of the diagnostic tools and more awareness of this clinical entity by physicians [2]. With respect to pulmonary neuroendocrine cells (PNECs), they have an endodermal origin, arising from stem cells of the bronchial epithelium known as Kulchitsky cells and are responsible of lung NETs (L-NETs), which represent about 25% of primary lung neoplasm, 10% of all carcinoid tumors are characterized as individual cells or small clusters (neuroepithelial bodies) [3]. From an epidemiological point of view, L-NETs occur more often during the fourth and fifth decades although they have been reported in every age group without any sex prevalence or external environmental toxin detected as a risk factor [4]. These tumors are usually asymptomatic, but clinical presentation may occur being mainly associated with bronchial obstruction, persistent cough, hemoptysis, wheezing and dyspnea with carcinoid syndrome that occurs in about 2% of cases, depending on which hormones are secreted [4]. L-NETscan can be classified into four subtypes: well-differentiated, low-grade typical carcinoids (TCs); well-differentiated, intermediate-grade, atypical carcinoids (ACs) that are slightly more prevalent in older population; poorly differentiated, high-grade large cell neuroendocrine carcinomas (LCNECs) and high-grade small cell lung cancers (SCLCs) [5]. In the update of 2015, in the classification of lung tumors, WHO decreed diagnostic criteria for NETs through histopathological features such as cell size, cell morphologic features, mitotic index, architectural growth patterns and presence of necrosis, assembling all four histologic variants of L-NETs (TCs, ACs, LCNECs, and SCLCs) into one category [6]. This innovation facilitates differential diagnosis since carcinoids were grouped separately from LCNECs and SCLCs in the 2004 WHO classification. LCNECs and SCLCs are classified to have a high tumor grade, whereas well-differentiated lung NETs can be classified as having either a low (TCs) or intermediate (ACs) tumor grade. The most important features to characterize L-NET subtypes are two: presence or absence of necrosis and the number of mitoses per two mm2 of viable area of tumor. TCs are defined as lung carcinoid tumors measuring at least 0.5 cm, with fewer than two mitoses per 2 mm2 of viable area of tumor and lacking necrosis, whereas ACs have two to 10 mitoses per 2 mm2 of viable area of tumor, with the presence of necrosis that is often focal. A grading scheme that classifies lung NETs as grade 1 (TCs), grade 2 (ACs), and grade 3 (SCLCs and LCNECs) is very helpful in guiding therapy. Patients with grade 1 or 2 tumors are generally treated with somatostatin analogs (SSAs) and other drugs, whereas patients with grade 3 tumors are treated with chemotherapy. Finally, in addition to the grading of L-NET, in order to establish the most suitable cancer treatment, it is necessary to know the proliferative index measured by Ki-67, an independent prognostic factor (Table 1).
Table 1.
Histologic characteristics of lung carcinoid tumors.
| - | TC | AC | LCNEC | SCLC |
|---|---|---|---|---|
| Necrosis | No | Yes | Yes | Yes |
| Mitotic activity (per 10 High Power Fields) | < 2 | 2-10 | > 10 | > 15-20 |
| Cytologic features | Small and polygonal uniform cell grouping in nests, cords or broad sheets separated by a prominent vascular stroma and numerous thin-walled blood vessels | Cytologic pleomorphism and higher nuclear-to- cytoplasmic ratios | Large cell size, polygonal shape, low nuclear-cytoplasmic ratio | Architecture of the tumor clusters is poorly preserved, with large areas of necrosis separating small islands of viable tumor |
| Immunohisto-chemical staining | Diffuse and homogeneous neurosecretory granules (neuron-specific enolase, chromogranin, and synaptophysin) | Chromogranin A, synaptophysin, and neural cell adhesion molecule (NCAM/ CD56) | Chromogranin A, synaptophysin, and neural cell adhesion molecule (NCAM/ CD56) | Neurosecretory granules such as chromogranin and synaptophysin are usually present, but these are fewer and smaller than those observed in carcinoid tumors. |
| Grading | Low | Intermediate | High | High |
Abbreviations: TC = Typical Carcinoid; AC= Atypical Carcinoid; LCNEC = Large Cell Neuro-Endocrine Carcinoma; SCLC = Small-Cell Lung Cancer.
2. Diagnostic imaging and therapy in the management of patients with L-NETS
In terms of imaging techniques, the diagnostic algorithm usually takes into consideration chest X-ray, contrast-enhanced CT and MRI, in which all tumors (TC, AC, LCNEC and SCLC) despite being significantly different for histological characteristics, clinical behavior and prognosis, show very similar radiologic features, such as central well-defined spherical nodule, bronchial narrowing, deformation or obstruction and calcifications [7]. In addition, they share the unique feature of over-expressing somatostatin receptors (SSTRs), with five known subtypes (SSTR1–SSTR5) and SSTR2 being the most prominent one in differentiated NETs [8-12]. In this context, nuclear medicine plays a crucial role in the detection and correct assessment of neoplastic functional status as it provides in vivo metabolic data related to the over- expression of SSTRs and to the amine pathway [13-16]. In fact, currently available radiopharmaceuticals for imaging NETs are based on these two characteristics and can be divided into two groups with the former including radiolabeled peptide analogs of natural hormones capable to detect over-expressed peptide receptors on the surface of NET cells and the latter based on radiolabeled amine precursors that image NET metabolism [17-19]. Moreover, traditional PET imaging with 18F-fluorodeoxyglucose(18F-FDG) may be used as well in the forms that are more aggressive and in case of poorly differentiated NETs due to their lower expression of SSTRs and higher glucose metabolism [20-22]. Therefore, nuclear medicine imaging techniques including somatostatin receptor, dopamine receptor and metabolic imaging of NETs offer higher sensitivity and specificity in diagnosis, staging, follow-up and with respect to SSTR-based studies prediction of response to peptide receptor radionuclide therapy (PRRT) in comparison with morphological imaging techniques such as CT and MRI [23-26]. From a therapeutic point of view, patients with L-NETs are usually treated with surgery in case of limited loco-regional disease, whereas carcinoid syndrome is treated with SSA to inhibit serotonin production which is responsible for diarrhea, flushing and wheezing; extended disease instead, is mainly treated either with PRRT or palliatively with chemotherapy and radiotherapy [27-29]. The major interest of nuclear medicine is associated with the possibility to connect radiopharmaceuticals for diagnosis and therapy, i.e. to adopt a theranostic model [30-33]. In this sense, somatostatin receptor scintigraphy (SRS) and DOTA-PET, the latter with higher diagnostic accuracy, are of the utmost importance throughout the staging process being capable not only to detect primary lesions but also to evaluate nodal involvement and/or metastatic spreading of L-NETs [34-36]. Subsequently, using β- or α emitters to label similar molecules, it is possible to perform targeted radiotherapy only in selected patients, namely those who show a high uptake at the diagnostic phase [37,39]. In this paper, we focused on SSTR-based imaging since SRS and PET/CT studies are the best options to detect well-differentiated L-NETs and to classify patients according to their SSTR density. Many radiolabeled somatostatin analogs are available for human use, with variable sensitivity and specificity since they differ in terms of radionuclides, chelators, and affinity for different SSTR subtypes [39-43]. The aim of this study is to compare the imaging performance of the recently developed 99mTc-labeled somatostatin analog, 99mTc-hydrazinonicotinyl-Tyr(3)-octreotide 99mTc-HYNIC-TOC [Tektrotyd®], with the “historic” gold standard In-diethylenediaminepentaacetic acid-D-Phe(1)-octreotide 111In-OCT [Octreoscan®] and the “new” gold standard 68Ga-DOTAPET (DOTATOC/DOTANOC/DOTATATE), to diagnose and characterize SSTR presence in L-NETs. Furthermore, we evaluated the diagnostic and prognostic role of 18F-FDG PET/CT in the same patients. The results of every patient were confirmed with histological analysis, in particular with the proliferation index (Ki67% or MIB1).
3. 111In-DTPA-Octreotide
For a long time, scintigraphic detection of somatostatin receptor cellular expression was obtained thanks to anIndium-labeled somatostatin analog (SSA) [44] that showed a high binding affinity for SSTRs, in particular for SSTR2 [45]. The synthetic addition of diethylene-triamine-pentaacetic acid (DTPA) to octreotide, provides four carboxylic groups (COOH) for the formation of metal-binding complexes, [46] forming the SSA Pentetreotide.111In-Pentreotide (Octreoscan®) is commercially available for imaging with the so-calledSRS, a sensitive method for the detection of neuroendocrine tumors, their metastases and the management of patients with NETs (Figs. 1 and 2) [47]. However, this radiopharmaceutical has several drawbacks mainly related to In physical characteristics, such as limited availability and high costs due to its production by cyclotrons, a medium γ-energy leading to suboptimal image resolution and relatively high radiation burden for the patient [48]. Moreover, from a practical point of view, it is not convenient for patients to undergo SPECT/CT across two days with an early acquisition at 4h post-injection and a delayed one 24–48h later, dual time procedure suggested to improve diagnostic accuracy [49, 50].
4. 99mTc-EDDA/HYNIC-TOC
More recently, Tektrotyd® (Polatom) was introduced into the market as a dry kit formulation with high affinity to SSTR2, lower to SSTR3 and SSTR5 [51] and its use has been approved in several countries across Europe for imaging of patients with L-NETs and other SSTR-positive tumors [52]. When compared to Octreoscan®, its advantages include:
Better physical characteristics of 99mTc respect to 111In, with the former that is more suited for gamma cameras and SPECT/CT imaging;
Shorter half-life (approximately 6 hours versus 2.8 days) that allows one-day imaging protocol without renouncing to kinetic studies for up to 24 hours;
Lower physiological liver and bowel uptake;
Lower radiation burden, so that higher dosage can be administered resulting in better image quality;
Lower costs and wider availability;
99mTc-EDDA/HYNIC-TOC could also represent a good alternative to 68Ga-DOTA-peptides (DOTA-TOC, DOTA-NOC, DOTA-TATE) in hospitals or centers where PET/CT or 68Ge/68Ga generators are not available [53]. The use of a SPECT/CT system significantly improves specificity and accuracy of SSTR SPECT-alone studies [54] since it reduces false positive and false negative results by positively affecting image quality, allowing anatomical localization and definition of morphological characteristics of scintigraphic focal uptakes [55]. Therefore a more accurate differential diagnosis of the most uncertain lesions may be obtained [56].
5. PET imaging: DOTA-Peptides and FDG
In centers with the availability of a PET/CT scanner the main diagnostic tool for SSTR-based imaging is PET/CT with DOTA-peptides, using radiopharmaceuticals such as 68Ga-DOTA-1-Nal3-octreotide (68Ga-DOTA-NOC), 68Ga-DOTA-Tyr3-octreotide (68Ga-DOTA-TOC) and 68Ga-DOTA-Tyr3-octreotate (68Ga -DOTA-TATE). When comparing characteristics of DOTA-PET/CT versus SRS it is clear that the former is much better than the latter for several reasons including the synthesis of 68Ga-DOTA-peptides that is easier and relatively inexpensive since 68Ga is produced via a specific 68Ge/68Ga generator, whereas production and labeling results are more expensive due to the need of a cyclotron [57].
In addition, from patient’s perspective, the whole study may be concluded in 2h instead of a dual-day imaging protocol with “early” 4h and“late” 24h acquisitions recommended with Octreoscan®, thus providing shorter examination times. On top of these pros, DOTA-peptides also have lower dosimetry and more favorable physical characteristics with higher spatial resolution compared to SPECT (3-6 mm versus 10-15 mm), which results in a significantly higher sensitivity and diagnostic accuracy [58]. PET is able to quantify tracer uptake in a given region of interest thanks to the standardized uptake value (SUVmax), which is useful to evaluate the therapeutic response and to be used as a prognostic factor. Finally, 68Ga-DOTA-peptides also present a higher affinity for SSTRs with a wider spectrum compared to 111In-Pentetreotide; for instance, 68Ga-DOTA-NOC binds to SSTR2, SSTR3 and SSTR5 compared to 111In-Pentetreotide that has high binding affinity only for SSTR2, whereas 68Ga-DOTA-TATE has the highest affinity for SSTR2 (about ten times higher) [59]. Reubi and Waser studied the distribution of SSTR in bronchial carcinoids and showed a prevalence of SSTR1 and SSTR2 (highest density), detected in 70% of tumors, while SSTR5 was found in 20% of cases and with lower density [60-62]. Therefore, the use of 68Ga-DOTA-TATE, SSTR2-specific radiotracer, above the others could be preferred, although no relevant clinical difference has been demonstrated yet among DOTA-peptides [17].
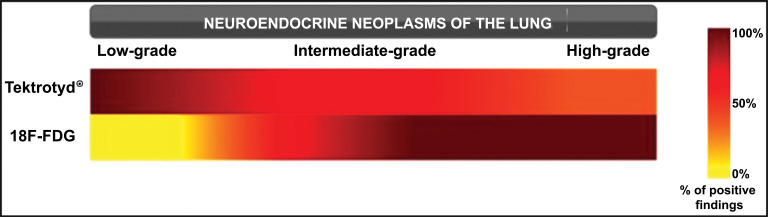
A metabolic imaging technique that could be used in L-NETs is 18F-FDG-PET/CT. In this case, the determination of malignant lesions is based on their glucose metabolism, with the over-expression of glucose transporter-1 (GLUT1) and increased glycolysis that has been shown to be closely related to 18F-FDG uptake in human cancer. However, in early-stage L-NETs, markers of glucose metabolism, hypoxia and angiogenesis determine the amount of 18F-FDG uptake [63]. Therefore, albeit the most diffused and employed PET tracer in oncology, 18F-FDG PET/CT, which is useful to characterize NSCLCs from a metabolic point of view, is affected by a low sensitivity when compared to SSTR-based imaging techniques in case of well-differentiated L-NETs and/or slow growing patterns that do not present an increased glucose metabolism. Therefore, its role could be limited to the detection and staging of high-grade tumors (LCNECs and SCLCs). Besides, since L-NETs 18F-FDG uptake (measured as the ratio between SUVmax and SUVliver) is related to Ki67 index, 18F-FDG-PET/CT is a valuable tool also in their metabolic characterization especially in ACs with higher proliferation index (Ki-67 index of 10%–20%), and in follow up of patients showing a prognostic worsening, related to de-differentiation (Figs. 3 and 4) [64].
6. Clinical role of 99mTc-HYNIC-TOC (Tektrotyd®) in the management of patients with L-NETs
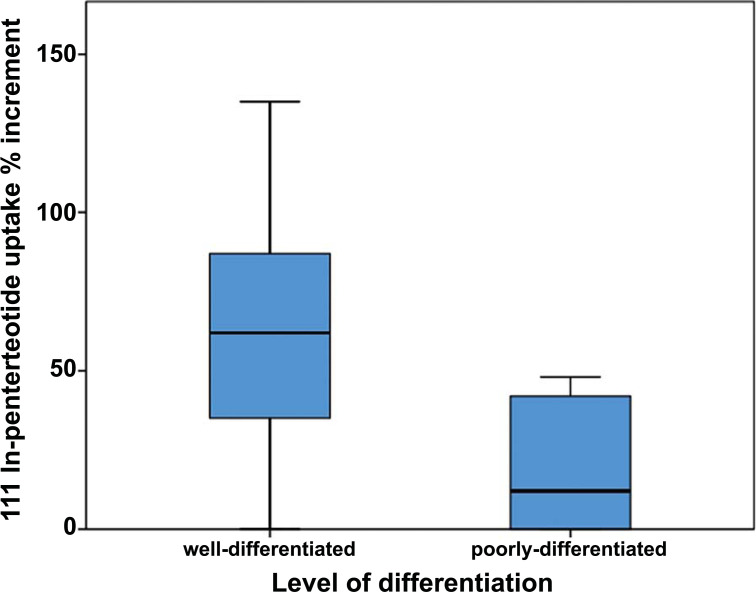
The correct definition of the clinical role of Tektrotyd® in the management of patients with L-NETs represents the main question due to the lack of sufficient data [52]. In fact, it is still not possible to consider Tektrotyd® superior to Octreoscan® although the first published papers that compare these two radiopharmaceuticals support the superiority of the former rather than the latter [53, 65]. Similarly, there are only a few comparisons between Tektrotyd® and DOTA-PET/CT [66]. In order to determine the possible clinical application of Tektrotyd®, as alternative or integrative technique for Octreoscan® and/or DOTA-PET/CT, it is important to understand the physical and biochemical characteristics of this tracer. Two main points are interesting, namely spatial resolution and tracer bio-distribution. With respect to the former, DOTA-PET/CT represents the best choice since it grants a minimum threshold of 4-5 mm, followed by Tektrotyd® that has a spatial resolution of 7-9 mm up to Octreoscan® that among the three has the lowest sensitivity with 11-14 mm. The other feature to evaluate is the possibility to study tracer’s bio-distribution over time, i.e. to study its pharmacokinetics and pharmacodynamics, in either a photographic or a cinematic mode. This is virtually impossible with 68Ga-DOTA-peptides since their washout rate is rather slow and radiopharmaceutical’s extraction is not too high, whereas Octreoscan® provides more information thanks to the delayed acquisitions either at 4 and 24 hours. In a similar fashion, Tektrotyd® can study biochemical distribution over time. In fact, it showed a comparable pattern of uptake to Octreoscan® with a tumor-non-tumor ratio (TNT) that an increase in the very first hours, in particular, more than 50% between 1h and 4h acquisitions [67]. Therefore, if Tektrotyd® proves to be not significantly inferior to DOTA-PET/CT in terms of diagnostic accuracy, thanks to the possibility of a kinetic analysis, it could represent the best choice in terms of cost/effectiveness in a large group of indications. In this sense, 111In-pentetreotide allows a thorough evaluation of bio-distribution kinetics but it is characterized by lower sensitivity, whereas99mTc-HYNIC-TOC has interesting intermediate features between Octreoscan® and DOTA-PET/CT (Fig. 5).
Fig. (5).
Tumor percentage increment (4h-24h) of 111In-Pentetreotide uptake, reflecting SSTR density, is higher in well-differentiated NETs (i.e. TC and AC) than in poorly-differentiated NETs (i.e. SCLC and LCNEC). 99mTc-HYNIC-TOC has the same uptake pattern of 111In-pentetreotide. Adapted from Education Exhibit presentation “Neuroendocrine Neoplasms of the Lung: Pathologic Classification and Spectrum of Molecular Imaging Findings”, RSNA Annual Meeting 2012. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
In 2014, Etchebehere et al. [68] prospectively compared 68Ga-DOTATATE PET/CT, 99mTc-HYNIC-Octreotide SPECT/CT and Whole-Body MRI in detection of NETs, suggesting a superiority of DOTA-PET in terms of diagnostic accuracy respect to the others for the detection of lesions in the pancreas, gastrointestinal tract, and skeletal system, but all three showed similar performances for lung, lymph node and liver lesions. MRI in the assessment of patients with L-NETs takes advantage of the vascular nature of the tumor; in fact, thanks to the intravenous administration of contrast media, lesions enhance intensely during the arterial phase and wash out during the delayed phase. Conversely, with respect to diffusion-weighted imaging, the tumor shows a high signal intensity because NETs significantly reduce water diffusion compared with normal tissues. In their study however, WB DWI was less sensitive than CT because sub-centimeter nodules were difficult to be detected on coronal images. Instead, 68Ga-DOTATATE PET/CT and SSRS SPECT/CT showed a high negative predictive value (NPV=0.88) with the absence of uptake in lung nodules that were considered to be dominant above CT; it means that nodules were considered negative even though they were suggestive for metastases from a morphological point of view. The only limitation they reported was related to false-negative results in lesions close to the liver dome because of respiratory motion artifacts, hence suggesting that respiratory gating could be an important tool to avoid interpretation errors at the level of diaphragmatic lesions.
Artiko et al. [69] instead, recently published the preliminary results of a multicenter trial that included 495 patients with different NETs studied with 99mTc-HYNIC-TOC SPECT/CT. With respect to L-NETs, there were 37 true positives, 6 true negatives, 1 false positive, and 6 false-negative results, resulting in 86% sensitivity, 85.7% specificity, 97.3% positive predictive value (PPV), 50% NPV and 86% accuracy.
Therefore, Tektrotyd could be more cost/effective in case of well-differentiated L-NETs, such as TCs and ACs, thanks to its comparable uptake with 111In-Pentetreotide with reduced radiation exposure, scanning time and with increased accuracy and sensitivity, especially with the current use of SPECT/CT. In fact, SRS SPECT/CT, by the combination of morphologic and metabolic data allows precise location of lesions thus reducing false-positive results.
More generally, although it is not possible to quantify the advantages and disadvantages in the comparison between Tektrotyd versus Octreoscan and 68Ga-peptides, we can, however, individuate better characteristics of each of them compared to the other ones. With respect to scan time and overall duration of patient examination, also affecting his/her compliance, DOTAPET is faster and takes less time, being concluded in 2 hours compared to Tektrotyd (5 hours) and Octreoscan, requiring a late scan at 24 hours. Being optimal for the diagnostic accuracy for DOTAPET, Tektrotyd allows a better signal/noise ratio and a higher counting rate with respect to Octreoscan that could determine (in a comparison involving a larger series of patients) better results. A major advantage for Tektrotyd may be connected with its wider availability, associated with the diffuse existence of 99mTc generators in all the departments worldwide, while 68Ga generators are only present in few centers, furthermore requiring the presence of a higher radiochemical competence. In this context, which could also significantly affect the cost/effectiveness because of different prices of the radioactive dose, Octreoscan is negatively charged by the need to purchase from the manufacturers. Although its faster physical decay represents a dosimetric advantage, 68Ga is negatively affected by the impossibility to carry out pharmacokinetic studies, feasible for Octreoscan and Tektrotyd, allowing scans up to 24 hrs (and also beyond for 111In). This feature may determine a better dosimetric evaluation of a theranostic effect, when associated with PRRT. Having been already demonstrated for Octreoscan the capability to in vivo differentiate NSCLC versus SCLC, because of a different washout at 24 hours, first data acquired by our group are in agreement with similar results achievable with Tektrotyd. Furthermore, only Octreoscan and Tektrotyd may allow a radioguided surgery, useful in operable pulmonary tumors, when in vivo concentrating radiolabeled Somatostatin analogs. In this context, although comparative studies are not available, a radiotracer labeled with 99mTc should allow a better signal to noise ratio respect to the corresponding radiopharmaceutical labeled with determining technical and methodological advantages in the intra-surgical staging. Therefore, although the diagnostic accuracy of DOTAPET still remains superior, a cost/effectiveness balance could favor the use of Tektrotyd in some indications either in oncology and for non oncological applications.
7. Methodology comparison in a rare disease: diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH)
This is a rare and poorly understood lung condition characterized by the abnormal overgrowth of PNECs, in the presence of neuroepithelial bodies in respiratory epithelium and multiple carcinoid tumorlets [70]. The cause is still unknown and since so few cases have been reported in the medical literature, there is limited information also on the prognosis and management of this condition. People with this diagnosis may have no obvious symptoms or may exhibit features of airway disease such as a chronic, non-productive cough, shortness of breath with exertion, and wheezing. It is considered a precancerous condition as the studies suggest that it is a precursor for TCs and ACs; in particular, a recent paper has proposed the minimum pathologic criteria necessary to diagnose DIPNECH as the presence of PNEC hyperplasia in at least three bronchioles associated with three or more tumorlets [71] (Fig. 6).
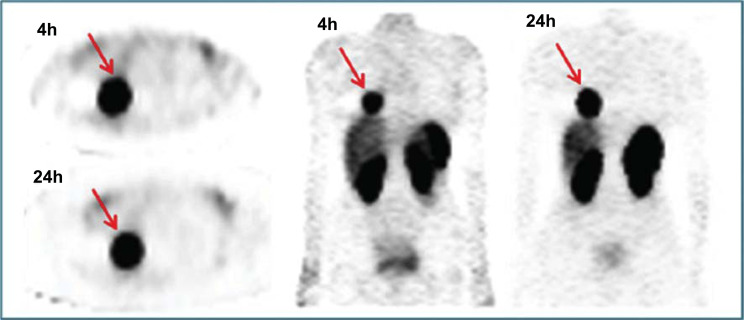
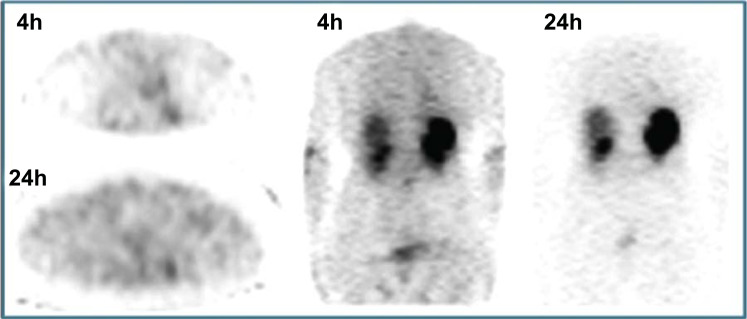
Fig. (6).
Comparison of FDG PET/CT, 4h Tektrotyd® and 24h Octreoscan® in 56yo female patient with history of DIPNECH and solitary nodule in the inferior lobe of the right lung. Moderately positive FDG PET/CT was less sensitive than SSTR-based SPECT imaging. Subsequent lobectomy confirmed the presence of TC (Ki67<1%). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
8. Discussion
Carcinoids are rare pulmonary tumors. The distinction between well-differentiated (TC and AC) and poorly differentiated (LCNEC e SCLC) and, also, between TC or AC, is clinically important, because it has therapeutic as well as prognostic implications. Typical carcinoid tumors are found to have a much better prognosis with a reported 5-year survival of 87-100% in contrast to the atypical carcinoids, which have a reported 5-year survival rate of 25-69% [72]. Imaging techniques play an important role in the identification of lung NETs, including key tumor characteristics, lymph node involvement and the presence of metastases, but they cannot define the different histopathology [73]. Carcinoids express SSTRs on their surface. Such receptors allow functional imaging of these tumors with radiolabelled somatostatin analogs like 68Ga-DOTATOC, 111In-Octreoscan® and 99mTc-Tektrotyd®. The density of these receptors is related to the degree of tumor differentiation, with TCs expressing the highest density and being the most well-differentiated ones [74]. In this paper, we tried to outline the main advantages and drawbacks of Tektrotyd® in comparison with Octreoscan®, 68Ga-DOTA-PET/CT and 18F-FDG-PET/CT studies in order to define the possible clinical role of the 99mTechnetium-based somatostatin analog, as a prognostic index for the characterization of lung NETs. In a recent meta-analysis, Treglia et al. [75] compared 16 studies including 567 patients with gastroenteropancreatic (GEP) and L-NETs, evaluated with 68Ga-DOTA-peptides PET/CT and SRS, reporting a significantly higher overall sensitivity and specificity for 68Ga-DOTA-peptides of 93% (95% confidence interval (CI): 91-95%) and 91% (95% CI: 82-97%), respectively. PET/MRI could represent a possible diagnostic improvement, which already demonstrated its potential in characterizing abdominal lesions GEP-NETs but not in case of L-NETs or hyper-sclerotic skeletal metastases (Beiderwellen KJ et al) [76]. (Fig. 7).
Fig. (7).
18F-FDG uptake (measured as the ratio between SUVmax and SUVliver) is related to Ki67 index in L-NETs. Adapted from Education Exhibit presentation “Neuroendocrine Neoplasms of the Lung: Pathologic Classification and Spectrum of Molecular Imaging Findings”, RSNA Annual Meeting 2012. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
In a different paper, Ambrosini et al. [17] studied 11 patients with pulmonary lesions, 10 subjects with pathologically proven TCs and one with highly suspicious CT images. In this series, 68Ga-DOTA-NOC was positive in nine patients with SUVmax ranging from 4.4 to 60.5 and thanks to the detection of unknown metastases, PET/CT was crucial to determine a change in the clinical management in three out of nine patients. In 2009, instead, Kayani et al. [22] compared 68Ga-DOTA-TATE and 18F-FDG PET/CT in 18 patients with L-NETs, and demonstrated the relationship between radiotracers uptake to tumor grade on histology. In particular,68Ga-DOTA-TATE showed an overall sensitivity of 72%, but the difference between the two tracers was more evident in case of TCs, where all of them showed high and selective 68Ga- DOTA-TATE uptake (SUVmax ≥ 8.2) compared to either negative or low 18F-FDG accumulation (SUVmax = 1.7–2.9). Conversely, all high-grade tumors and ACs showed high 18F-FDG uptake (SUVmax ≥ 11.7), versus a minimally increased uptake of 68Ga-DOTA-TATE (SUVmax = 2.2–2.8). Neither 68Ga-DOTA-TATE nor 18F-FDG uptake was observed in the case of DIPNECH.
In this context, in the near future, theranostic imaging of SSTRs will become the most prominent indication for SSTR-based techniques, including SRS, SPECT/CT and PET/CT, in order to document sufficient levels of SSTRs before suggesting a PRRT. In fact, great strides have been made to study and improve the performance of PRRT, which can be done mainly with 90Y or 177Lu-labeled DOTA-peptides, namely 90Y-DOTA-TOC and 177Lu-DOTA-TATE. Even if very few data, relative to their use in L-NETS, is available, these two radiopharmaceuticals already showed promising results being at the same time well tolerated and an effective therapeutic tool, being renal toxicity the only significant drawback. Therefore, in patients suitable for PRRT, GFR has to be monitored and, if possible, in order to reduce the renal absorbed dose, an i.v. infusion of positively charged aminoacids should be administered before therapy [27, 28].
Mariniello et al. [77] retrospectively analyzed 114 patients with advanced stage bronchopulmonary carcinoids from 1997 to 2012, treated with three different PRRT protocols, namely 177Lu-octreotate, 90Y-DOTATOC or the combination of both. In their study, despite the differences in treatment schemes and inter-individual diversity, 177Lu-octreotate seemed superior to 90Y-DOTATOC with morphological responses (partial responses + minor responses) that were obtained in 26.5% of the cohort and were associated with longer OS and PFS. More recently, Sabet et al. [78] engineered a dual-center retrospective study aimed to assess the outcome and toxicity of standardized PRRT with 177Lu-octreotate in 22 patients with G1-G2 advanced pulmonary NETs, after failing standard treatment with “cold” somatostatin analogs. Their results showed a partial response in six patients, corresponding to 27.3% of cases, indicating that 177Lu-octreotate anti-proliferative activity is present in this specific NET entity at an advanced stage, although the particular benefit in patients with L-NETs is still unclear, even more, if we consider small population sizes and the retrospective nature of the abovementioned papers.
Conclusion and Perspective
Although there are few papers comparing these 3 different approaches, we can conclude that in SSTR characterization of lung NETs, Tektrotyd® SPECT/CT apparently demonstrates at least the same capability as compared to Octreoscan®, remaining 68Ga-DOTATOC PET/CT the gold standard. In particular, when compared to 111In-Pentetreotide, Tektrotyd® showed a slightly higher sensitivity, in the presence of higher imaging quality and lower radiation exposure for patients. Furthermore, revealing typical carcinoids higher uptake on Tektrotyd® compared with atypical and poorly-differentiated tumors, also this radiopharmaceutical may be used for prognostic purposes.
In our opinion, Tektrotyd®, mainly when DOTAPET is not available, can be proposed for all indications associated with radiolabeled somatostatin analogs (SAA) in lung NETs, including not only diagnosis but also prognostic stratification and recruitment of patients undergoing therapy with SAA.
99mTc-HYNIC-TOC already represents a useful imaging radiotracer that can be used in clinical practice as an alternative diagnostic tool with respect to 111In-Pentetreotide. Similarly, it could be utilized as an alternative to 68Ga-DOTATOC in centers without the availability of a PET/CT scanner and/or without a 68Ge-68Ga generator.
Even using Tektrotyd®, T/NT ratio increase over time has been confirmed as a valuable tool in receptor characterization, being connected with a specific tumor uptake, associated with SSTR density on neoplastic cells. This behavior may improve confidence in diagnostic and prognostic analysis in lung NETs, adding useful information for a personalized dosimetric evaluation in patients undergoing radio-receptor therapy.
Although it is not strictly the field of interest of this paper, concerning NET lung tumors, the most interesting perspectives depending on the kinetic analysis allowed by Tektrotyd® could be obtained in the differential diagnosis of non-small cells lung cancer (NSCLC) versus small cells lung cancer (SCLC) and NETs. It has been demonstrated that NSCLC doesn’t express in vitro SSTRs on the cellular membrane, differently from SCLC and NETs. Conversely, NSCLC shows an in vivo uptake, associated with activated reactive cells surrounding the neoplasm. Using Octreoscan®, while an increased or stable T/NT ratio is observed in patients with SCLC and NETs, a lower ratio is seen at the delayed scan in NSCLC, due to the wash-out of the activity linked with mobile normal cells.
In agreement with indications suggested for Octreoscan®, also Tektrotyd® could be proposed in evaluating activity in pulmonary benign diseases such as sarcoidosis. Similarly, very effective in directing a surgical strategy and/or intraoperative staging, could be a surgery radioguided by Tektrotyd® in operable lung tumors, including either NETs and NSCLC [79].
To conclude this review, we further confirmed that also in lung NETs, the uptake of 18F-FDG tended to increase from low-grade to high-grade PNETs (Fig. 7). Therefore, 18F-FDG, non-indicated in the diagnosis of NETs because of its low sensitivity, may be suggested for prognostic stratification in the staging of patients in which an un-differentiation is suspected and re-staging of patients in which a de-differentiation is suspected.
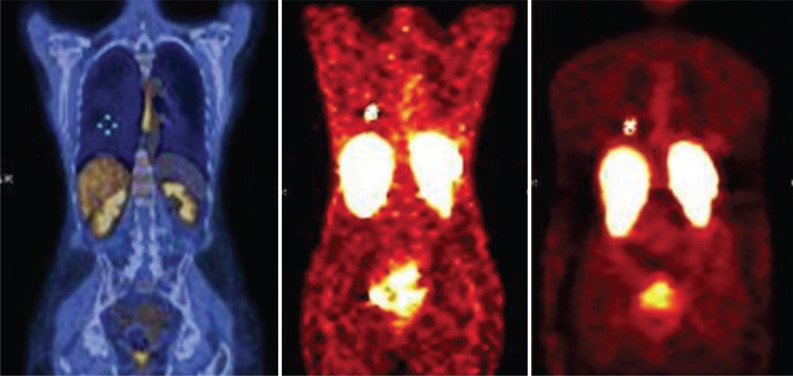
Fig. (1).
Octreoscan® SPECT in patient with TC of the right lung (M, 70 y.o.). Image shows high tumor uptake due to high SSTR density, with consistent (>50%) increase at delayed examinations. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (2).
Octreoscan® SPECT in patient with SCLC (M, 45 y.o.) that shows the usually low tracer uptake, which only sometimes can be intermediate or high. The evaluation of SSTR density is crucial for prediction of treatment response. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (3).
Comparison of percentage of positive findings between Tektrotyd® and 18F- FDG based on tumor grade. Adapted from Education Exhibit presentation “Neuroendocrine Neoplasms of the Lung: Pathologic Classification and Spectrum of Molecular Imaging Findings”, RSNA Annual Meeting 2012. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (4).
Transaxial FDG PET/CT in patient with SCLC (M, 39 y.o.) that shows high uptake within the tumor. This technique allows accurate tumor staging in particular for separation of limited disease versus extended disease. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Reid M.D., Balci S., Saka B., Adsay N.V. Neuroendocrine tumors of the pancreas: current concepts and controversies. Endocr. Pathol. 2014;25(1):65–79. doi: 10.1007/s12022-013-9295-2. [DOI] [PubMed] [Google Scholar]
- 2.Woltering E.A. American Joint Committee on Cancer 2017. In: Amin M.B., editor. AJCC Cancer Staging Manual. 8th ed. Springer; 2017. pp. 351–359. [Google Scholar]
- 3.Cameselle-Teijeiro J.M., Mato Mato J.A., Fernández Calvo O., García Mata J. Neuroendocrine Pulmonary Tumors of Low, Intermediate and High Grade: Anatomopathological Diagnosis-Prognostic and Predictive Factors. Mol. Diagn. Ther. 2018;22(2):169–177. doi: 10.1007/s40291-018-0315-2. [DOI] [PubMed] [Google Scholar]
- 4.Pelosi G., Sonzogni A., Harari S., Albini A., Bresaola E., Marchiò C., Massa F., Righi L., Gatti G., Papanikolaou N., Vijayvergia N., Calabrese F., Papotti M. Classification of pulmonary neuroendocrine tumors: new insights. Transl. Lung Cancer Res. 2017;6(5):513–529. doi: 10.21037/tlcr.2017.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filosso P.L., Falcoz P.E., Solidoro P., Pellicano D., Passani S., Guerrera F., Ruffini E. ESTS Lung Neuroendocrine Working-Group Participating Centers*. The European Society of Thoracic Surgeons (ESTS) lung neuroendocrine tumors (NETs) database. J. Thorac. Dis. 2018;10(Suppl. 29):S3528–S3532. doi: 10.21037/jtd.2018.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J.Y., Hong S.M., Ro J.Y. Recent updates on grading and classification of neuroendocrine tumors. Ann. Diagn. Pathol. 2017;29:11–16. doi: 10.1016/j.anndiagpath.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs M.A., Weinstein S., Hope T.A., Aslam R., Yee J., Coakley F. Neuroendocrine tumors: beyond the abdomen. J. Comput. Assist. Tomogr. 2014;38(6):898–914. doi: 10.1097/RCT.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 8.Cuccurullo V., Faggiano A., Scialpi M., Cascini G.L., Piunno A., Catalano O., Colao A., Mansi L. Questions and answers: what can be said by diagnostic imaging in neuroendocrine tumors? Minerva Endocrinol. 2012;37(4):367–377. [PubMed] [Google Scholar]
- 9.Reubi J.C., Waser B., Schaer J.C., Laissue J.A. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur. J. Nucl. Med. 2001;28(7):836–846. doi: 10.1007/s002590100541. [DOI] [PubMed] [Google Scholar]
- 10.Reubi J.C., Laissue J.A., Waser B., Steffen D.L., Hipkin R.W., Schonbrunn A. Immunohistochemical detection of somatostatin sst2a receptors in the lymphatic, smooth muscular, and peripheral nervous systems of the human gastrointestinal tract: facts and artifacts. J. Clin. Endocrinol. Metab. 1999;84(8):2942–2950. doi: 10.1210/jcem.84.8.5878. [DOI] [PubMed] [Google Scholar]
- 11.Desai H., Borges-Neto S., Wong T.Z. Molecular Imaging and Therapy for Neuroendocrine Tumors. Curr. Treat. Options Oncol. 2019;20(10):78. doi: 10.1007/s11864-019-0678-6. [DOI] [PubMed] [Google Scholar]
- 12.Krenning E.P., de Jong M., Kooij P.P., Breeman W.A., Bakker W.H., de Herder W.W., van Eijck C.H., Kwekkeboom D.J., Jamar F., Pauwels S., Valkema R. Radiolabelled somatostatin analogue(s) for peptide receptor scintigraphy and radionuclide therapy. Ann. Oncol. 1999;10(2):S23–S29. doi: 10.1093/annonc/10.suppl_2.s23. [DOI] [PubMed] [Google Scholar]
- 13.Mansi L., Cuccurullo V. Diagnostic imaging in neuroendocrine tumors. J. Mucl. Med. 2014;55(10):1576–1577. doi: 10.2967/jnumed.114.147082. [DOI] [PubMed] [Google Scholar]
- 14.Cuccurullo V., Prisco M.R., Di Stasio G.D., Mansi L. Nuclear Medicine in Patients with NET: Radiolabeled Somatostatin Analogues and their Brothers. Curr. Radiopharm. 2017;10(2):74–84. doi: 10.2174/1874471010666170323115136. [DOI] [PubMed] [Google Scholar]
- 15.Rufini V., Calcagni M.L., Baum R.P. Imaging of neuroendocrine tumors. Semin. Nucl. Med. 2006;36(3):228–247. doi: 10.1053/j.semnuclmed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Cascini G.L., Cuccurullo V., Tamburrini O., Rotondo A., Mansi L. Peptide imaging with somatostatin analogues: more than cancer probes. Curr. Radiopharm. 2013;6(1):36–40. doi: 10.2174/1874471011306010006. [DOI] [PubMed] [Google Scholar]
- 17.Ambrosini V., Morigi J.J., Nanni C., Castellucci P., Fanti S. Current status of PET imaging of neuroendocrine tumours ([18F]FDOPA, [68Ga]tracers, [11C]/[18F]-HTP). The Quarterly J. Mucl. Med. Mol. Imaging. 2015;59(1):58–69. [PubMed] [Google Scholar]
- 18.Cascini G.L., Cuccurullo V., Mansi L. The non tumour uptake of (111)In-octreotide creates new clinical indications in benign diseases, but also in oncology. Q. J. Nucl. Med. Mol. Imaging. 2010;54(1):24–36. [PubMed] [Google Scholar]
- 19.Cuccurullo V., Mansi L. Toward tailored medicine (and beyond): the phaeochromocytoma and paraganglioma model. Eur. J. Nucl. Med. Mol. Imaging. 2012;39(8):1262–1265. doi: 10.1007/s00259-012-2156-2. [DOI] [PubMed] [Google Scholar]
- 20.Ambrosini V., Nanni C., Fanti S. The use of gallium-68 labeled somatostatin receptors in PET/CT imaging. PET Clin. 2014;9(3):323–329. doi: 10.1016/j.cpet.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Cuccurullo V., Di Stasio G.D., Evangelista L., Castoria G., Mansi L. Biochemical and pathophysiological premises to positron emission tomography with choline radiotracers. J. Cell. Physiol. 2017;232(2):270–275. doi: 10.1002/jcp.25478. [DOI] [PubMed] [Google Scholar]
- 22.Kayani I., Conry B. G., Groves A. M., Win T., Dickson J., Caplin M., Bomanji J. B. 2009. [DOI] [PubMed]
- 23.Cuccurullo V., Di Stasio G.D., Prisco M.R., Mansi L. Is there a clinical usefulness for radiolabeled somatostatin analogues beyond the consolidated role in NETs? Indian J. Radiol. Imaging. 2017;27(4):509–516. doi: 10.4103/ijri.IJRI_431_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Binderup T., Knigge U., Loft A., Mortensen J., Pfeifer A., Federspiel B., Hansen C. P., Hojgaard L., Kjaer A. 2010. [DOI] [PubMed]
- 25.Briganti V., Cuccurullo V., Di Stasio G.D., Mansi L. Gamma emitters in pancreatic endocrine tumors imaging: is there a clinical space for 99mTc-peptides? Curr. Radiopharm. 2019;12(2):156–170. doi: 10.2174/1874471012666190301122524. [DOI] [PubMed] [Google Scholar]
- 26.Cuccurullo V., Di Stasio G.D., Mansi L. Physiopathological premises to Nuclear Medicine Imaging and therapy of pancreatic neuroendocrine tumours. Curr. Radiopharm. doi: 10.2174/1874471012666190206094555. In Press. [DOI] [PubMed] [Google Scholar]
- 27.Bodei L., Ferone D., Grana C.M., Cremonesi M., Signore A., Dierckx R.A., Paganelli G. Peptide receptor therapies in neuroendocrine tumors. J. Endocrinol. Invest. 2009;32(4):360–369. doi: 10.1007/BF03345728. [DOI] [PubMed] [Google Scholar]
- 28.Virgolini I., Innsbruck T. Innsbruck Team. Peptide receptor radionuclide therapy (PRRT): clinical significance of re-treatment? Eur. J. Nucl. Med. Mol. Imaging. 2015;42(13):1949–1954. doi: 10.1007/s00259-015-3153-z. [DOI] [PubMed] [Google Scholar]
- 29.Bodei L., Cremonesi M., Kidd M., Grana C.M., Severi S., Modlin I.M., Paganelli G. Peptide receptor radionuclide therapy for advanced neuroendocrine tumors. Thorac. Surg. Clin. 2014;24(3):333–349. doi: 10.1016/j.thorsurg.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Kitson S.L., Cuccurullo V., Moody T.S., Mansi L. Radionuclide antibody-conjugates, a targeted therapy towards cancer. Curr. Radiopharm. 2013;6(2):57–71. doi: 10.2174/1874471011306020001. [DOI] [PubMed] [Google Scholar]
- 31.Cascini G.L., Cuccurullo V., Tamburrini O., Mansi L., Rotondo A. Nuclear medicine in multiple myeloma -- more than diagnosis. Nucl. Med. Rev. Cent. East. Eur. 2010;13(1):32–38. [PubMed] [Google Scholar]
- 32.Mansi L., Cuccurullo V., Ciarmiello A. From Homo sapiens to Homo in nexu (connected man): could functional imaging redefine the brain of a “new human species”? Eur. J. Nucl. Med. Mol. Imaging. 2014;41(7):1385–1387. doi: 10.1007/s00259-014-2765-z. [DOI] [PubMed] [Google Scholar]
- 33.Baum R.P., Kulkarni H.R., Carreras C. Peptides and receptors in image-guided therapy: theranostics for neuroendocrine neoplasms. Semin. Nucl. Med. 2012;42(3):190–207. doi: 10.1053/j.semnuclmed.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Kwekkeboom D.J., Kam B.L., van Essen M., Teunissen J.J., van Eijck C.H., Valkema R., de Jong M., de Herder W.W., Krenning E.P. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr. Relat. Cancer. 2010;17(1):R53–R73. doi: 10.1677/ERC-09-0078. [DOI] [PubMed] [Google Scholar]
- 35.Upadhyay B., Lu S.J., Navalkissoor S., Gnanasegaran G., Buscombe J. The imaging of neuroendocrine tumors using single photon emission computed tomography/computed tomography. The quarterly J. Mucl. Med. Mol. Imaging. 2015;59(2):140–151. [PubMed] [Google Scholar]
- 36.Win Z., Al-Nahhas A., Rubello D., Gross M.D. Somatostatin receptor PET imaging with Gallium-68 labeled peptides. The quarterly J. Mucl. Med. Mol. Imaging. 2007;51(3):244–250. [PubMed] [Google Scholar]
- 37.Bakker W.H., Breeman W.A., Kwekkeboom D.J., De Jong L.C., Krenning E.P. Practical aspects of peptide receptor radionuclide therapy with [177Lu][DOTA0, Tyr3]octreotate. The Quarterly J. Mucl. Med. Mol. Imaging. 2006;50(4):265–271. [PubMed] [Google Scholar]
- 38.Kitson S.L., Cuccurullo V., Ciarmiello A., Mansi L. Targeted Therapy Towards Cancer-A Perspective. Anticancer. Agents Med. Chem. 2017;17(3):311–317. doi: 10.2174/1871520616666160926115008. [DOI] [PubMed] [Google Scholar]
- 39.Mikołajczak R., Maecke H.R. Radiopharmaceuticals for somatostatin receptor imaging. Nucl. Med. Rev. Cent. East. Eur. 2016;19(2):126–132. doi: 10.5603/NMR.2016.0024. [DOI] [PubMed] [Google Scholar]
- 40.Cuccurullo V., Di Stasio G.D., Schillirò M.L., Mansi L. Small-Animal Molecular Imaging for Preclinical Cancer Research. μPET and μ.SPECT. Curr. Radiopharm. 2016;9(2):102–113. doi: 10.2174/1874471008666151027154148. [DOI] [PubMed] [Google Scholar]
- 41.Maina T., Cescato R., Waser B., Tatsi A., Kaloudi A., Krenning E.P., de Jong M., Nock B.A., Reubi J.C. [111In-DOTA]LTT-SS28, a first pansomatostatin radioligand for in vivo targeting of somatostatin receptor-positive tumors. J. Med. Chem. 2014;57(15):6564–6571. doi: 10.1021/jm500581d. [DOI] [PubMed] [Google Scholar]
- 42.Tatsi A., Maina T., Cescato R., Waser B., Krenning E.P., de Jong M., Cordopatis P., Reubi J.C., Nock B.A. [DOTA]Somatostatin-14 analogs and their (111)In-radioligands: effects of decreasing ring-size on sst1-5 profile, stability and tumor targeting. Eur. J. Med. Chem. 2014;73:30–37. doi: 10.1016/j.ejmech.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Sowa-Staszczak A., Pach D., Mikołajczak R., Mäcke H., Jabrocka-Hybel A., Stefańska A., Tomaszuk M., Janota B., Gilis-Januszewska A., Małecki M., Kamiński G., Kowalska A., Kulig J., Matyja A., Osuch C., Hubalewska-Dydejczyk A. Glucagon-like peptide-1 receptor imaging with [Lys40(Ahx-HYNIC- 99mTc/EDDA)NH2]-exendin-4 for the detection of insulinoma. Eur. J. Nucl. Med. Mol. Imaging. 2013;40(4):524–531. doi: 10.1007/s00259-012-2299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reubi J.C. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr. Rev. 2003;24(4):389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 45.Reubi J.C., Schär J.C., Waser B., Wenger S., Heppeler A., Schmitt J.S., Mäcke H.R. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000;27(3):273–282. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 46.Smit Duijzentkunst D.A., Kwekkeboom D.J., Bodei L. Somatostatin Receptor 2-Targeting Compounds. J. Nucl. Med. 2017;58(Suppl. 2):54S–60S. doi: 10.2967/jnumed.117.191015. [DOI] [PubMed] [Google Scholar]
- 47.van der Lely A.J., de Herder W.W., Krenning E.P., Kwekkeboom D.J. Octreoscan radioreceptor imaging. Endocrine. 2003;20(3):307–311. doi: 10.1385/ENDO:20:3:307. [DOI] [PubMed] [Google Scholar]
- 48.Cuccurullo V., Cascini G.L., Tamburrini O., Mansi L., Rotondo A. Less frequent requests for In-111 pentreotide and its brothers of endocrinological interest. Minerva Endocrinol. 2011;36(1):41–52. [PubMed] [Google Scholar]
- 49.Goldsmith S.J. Update on nuclear medicine imaging of neuroendocrine tumors. Future Oncol. 2009;5(1):75–84. doi: 10.2217/14796694.5.1.75. [DOI] [PubMed] [Google Scholar]
- 50.Briganti V., Matteini M., Ferri P., Vaggelli L., Castagnoli A., Pieroni C. Octreoscan SPET evaluation in the diagnosis of pancreas neuroendocrine tumors. Cancer Biother. Radiopharm. 2001;16(6):515–524. doi: 10.1089/10849780152752119. [DOI] [PubMed] [Google Scholar]
- 51.Wang F., Wang Z., Yao W., Xie H., Xu J., Tian L. Role of 99mTc-octreotide acetate scintigraphy in suspected lung cancer compared with 18F-FDG dual-head coincidence imaging. J. Nucl. Med. 2007;48(9):1442–1448. doi: 10.2967/jnumed.107.040824. [DOI] [PubMed] [Google Scholar]
- 52.Czepczyński R., Parisella M.G., Kosowicz J., Mikołajczak R., Ziemnicka K., Gryczyńska M., Sowiński J., Signore A. Somatostatin receptor scintigraphy using 99mTc-EDDA/HYNIC-TOC in patients with medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging. 2007;34(10):1635–1645. doi: 10.1007/s00259-007-0479-1. [DOI] [PubMed] [Google Scholar]
- 53.Artiko V., Sobic-Saranovic D., Pavlovic S., Petrovic M., Zuvela M., Antic A., Matic S., Odalovic S., Petrovic N., Milovanovic A., Obradovic V. The clinical value of scintigraphy of neuroendocrine tumors using (99m)Tc-HYNIC-TOC. J. BUON. 2012;17(3):537–542. [PubMed] [Google Scholar]
- 54.Trogrlic M., Težak S. Incremental value of 99mTc-HYNIC-TOC SPECT/CT over whole-body planar scintigraphy and SPECT in patients with neuroendocrine tumours. Nucl. Med. (Stuttg.) 2017;56(3):97–107. doi: 10.3413/Nukmed-0851-16-10. [DOI] [PubMed] [Google Scholar]
- 55.Yamaga L.Y., Neto G.C., da Cunha M.L., Osawa A., Oliveira J.C., Fonseca R.Q., Nogueira S.A., Wagner J., Funari M.G. 99mTc-HYNIC-TOC increased uptake can mimic malignancy in the pancreas uncinate process at somatostatin receptor SPECT/CT. Radiol. Med. (Torino) 2016;121(3):225–228. doi: 10.1007/s11547-015-0593-2. [DOI] [PubMed] [Google Scholar]
- 56.Cuccurullo V., Cascini G.L., Tamburrini O., Rotondo A., Mansi L. Bone metastases radiopharmaceuticals: an overview. Curr. Radiopharm. 2013;6(1):41–47. doi: 10.2174/1874471011306010007. [DOI] [PubMed] [Google Scholar]
- 57.Velikyan I. 68Ga-Based radiopharmaceuticals: production and application relationship. Molecules. 2015;20(7):12913–12943. doi: 10.3390/molecules200712913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kulkarni H.R., Baum R.P. Theranostics with Ga-68 somatostatin receptor PET/CT: monitoring response to peptide receptor radionuclide therapy. PET Clin. 2014;9(1):91–97. doi: 10.1016/j.cpet.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 59.Eberlein U., Lassmann M. Dosimetry of [(6)(8)Ga]-labeled compounds. Appl. Radiat. Isot. 2013;76:70–74. doi: 10.1016/j.apradiso.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 60.Reubi J.C., Waser B., Laissue J.A., Gebbers J-O. Somatostatin and vasoactive intestinal peptide receptors in human mesenchymal tumors: in vitro identification. Cancer Res. 1996;56(8):1922–1931. [PubMed] [Google Scholar]
- 61.Reubi J.C., Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur. J. Nucl. Med. Mol. Imaging. 2003;30(5):781–793. doi: 10.1007/s00259-003-1184-3. [DOI] [PubMed] [Google Scholar]
- 62.Barnett P. Somatostatin and somatostatin receptor physiology. Endocrine. 2003;20(3):255–264. doi: 10.1385/ENDO:20:3:255. [DOI] [PubMed] [Google Scholar]
- 63.Beyer T., Hacker M., Schubiger A., Virgolini I., Wester H.J. Nuclear medicine 2013: from status quo to status go. Eur. J. Nucl. Med. Mol. Imaging. 2013;40(12):1794–1796. doi: 10.1007/s00259-013-2570-0. [DOI] [PubMed] [Google Scholar]
- 64.Del Gobbo A., Pellegrinelli A., Gaudioso G., Castellani M., Zito Marino F., Franco R., Palleschi A., Nosotti M., Bosari S., Vaira V., Ferrero S. Analysis of NSCLC tumour heterogeneity, proliferative and 18F-FDG PET indices reveals Ki67 prognostic role in adenocarcinomas. Histopathology. 2016;68(5):746–751. doi: 10.1111/his.12808. [DOI] [PubMed] [Google Scholar]
- 65.Płachcińska A., Mikołajczak R., Kozak J., Rzeszutek K., Kuśmierek J. Comparative analysis of 99mTc-depreotide and 99mTc-EDDA/HYNIC-TOC thorax scintigrams acquired for the purpose of differential diagnosis of solitary pulmonary nodules. Nucl. Med. Rev. Cent. East. Eur. 2006;9(1):24–29. [PubMed] [Google Scholar]
- 66.Kunikowska J., Lewington V., Krolicki L. Optimizing somatostatin receptor imaging in patients with neuroendocrine tumors: The impact of 99mTc-HYNICTOC SPECT/SPECT/CT versus 68Ga-DOTATATE PET/CT upon clinical management. Clin. Nucl. Med. 2017;42(12):905–911. doi: 10.1097/RLU.0000000000001877. [DOI] [PubMed] [Google Scholar]
- 67.Briganti V., Sestini R., Orlando C., Bernini G., La Cava G., Tamburini A., Raggi C.C., Serio M., Maggi M. Imaging of somatostatin receptors by indium-111-pentetreotide correlates with quantitative determination of somatostatin receptor type 2 gene expression in neuroblastoma tumors. Clin. Cancer Res. 1997;3(12 Pt 1):2385–2391. [PubMed] [Google Scholar]
- 68.Etchebehere E.C., de Oliveira Santos A., Gumz B., Vicente A., Hoff P.G., Corradi G., Ichiki W.A., de Almeida Filho J.G., Cantoni S., Camargo E.E., Costa F.P. 68Ga-DOTATATE PET/CT, 99mTc-HYNIC-octreotide SPECT/CT, and whole-body MR imaging in detection of neuroendocrine tumors: a prospective trial. J. Nucl. Med. 2014;55(10):1598–1604. doi: 10.2967/jnumed.114.144543. [DOI] [PubMed] [Google Scholar]
- 69.Artiko V., Afgan A., Petrović J., Radović B., Petrović N., Vlajković M., Šobić-Šaranović D., Obradović V. Evaluation of neuroendocrine tumors with 99mTc-EDDA/HYNIC TOC. Nucl. Med. Rev. Cent. East. Eur. 2016;19(2):99–103. doi: 10.5603/NMR.2016.0020. [DOI] [PubMed] [Google Scholar]
- 70.Rossi G., Cavazza A., Spagnolo P., Sverzellati N., Longo L., Jukna A., Montanari G., Carbonelli C., Vincenzi G., Bogina G., Franco R., Tiseo M., Cottin V., Colby T.V. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia syndrome. Eur. Respir. J. 2016;47(6):1829–1841. doi: 10.1183/13993003.01954-2015. [DOI] [PubMed] [Google Scholar]
- 71.Koliakos E., Thomopoulos T., Abbassi Z., Duc C., Christodoulou M. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: a case report and review of the literature. Am. J. Case Rep. 2017;18:975–979. doi: 10.12659/AJCR.904468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Melosky B. Advanced typical and atypical carcinoid tumours of the lung: management recommendations. Curr. Oncol. 2018;25(Suppl. 1):S86–S93. doi: 10.3747/co.25.3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramirez R.A., Chauhan A., Gimenez J., Thomas K.E.H., Kokodis I., Voros B.A. Management of pulmonary neuroendocrine tumors. Rev. Endocr. Metab. Disord. 2017;18(4):433–442. doi: 10.1007/s11154-017-9429-9. [DOI] [PubMed] [Google Scholar]
- 74.Bodei L., Ćwikla J.B., Kidd M., Modlin I.M. The role of peptide receptor radionuclide therapy in advanced/metastatic thoracic neuroendocrine tumors. J. Thorac. Dis. 2017;9(Suppl. 15):S1511–S1523. doi: 10.21037/jtd.2017.09.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Treglia G., Kroiss A.S., Piccardo A., Lococo F., Santhanam P., Imperiale A. Role of positron emission tomography in thyroid and neuroendocrine tumours. Minerva Endocrinol. 2017;•••:S0391–S1977. doi: 10.23736/S0391-1977.17.02742-0. [DOI] [PubMed] [Google Scholar]
- 76.Beiderwellen K.J., Poeppel T.D., Hartung-Knemeyer V., Buchbender C., Kuehl H., Bockisch A., Lauenstein T.C. Simultaneous 68Ga-DOTATOC PET/MRI in patients with gastroenteropancreatic neuroendocrine tumors: initial results. Invest. Radiol. 2013;48(5):273–279. doi: 10.1097/RLI.0b013e3182871a7f. [DOI] [PubMed] [Google Scholar]
- 77.Mariniello A., Bodei L., Tinelli C., Baio S.M., Gilardi L., Colandrea M., Papi S., Valmadre G., Fazio N., Galetta D., Paganelli G., Grana C.M. Long-term results of PRRT in advanced bronchopulmonary carcinoid. Eur. J. Nucl. Med. Mol. Imaging. 2016;43(3):441–452. doi: 10.1007/s00259-015-3190-7. [DOI] [PubMed] [Google Scholar]
- 78.Sabet A., Haug A.R., Eiden C., Auernhammer C.J., Simon B., Bartenstein P., Biersack H.J., Ezziddin S. Efficacy of peptide receptor radionuclide therapy with 177Lu-octreotate in metastatic pulmonary neuroendocrine tumors: a dual-centre analysis. Am. J. Nucl. Med. Mol. Imaging. 2017;7(2):74–83. [PMC free article] [PubMed] [Google Scholar]
- 79.Cuccurullo V., Di Stasio G.D., Mansi L. Radioguided surgery with radiolabeled somatostatin analogs: not only in GEP-NETs. Nucl. Med. Rev. Cent. East. Eur. 2017;20(1):49–56. doi: 10.5603/NMR.2017.0003. [DOI] [PubMed] [Google Scholar]