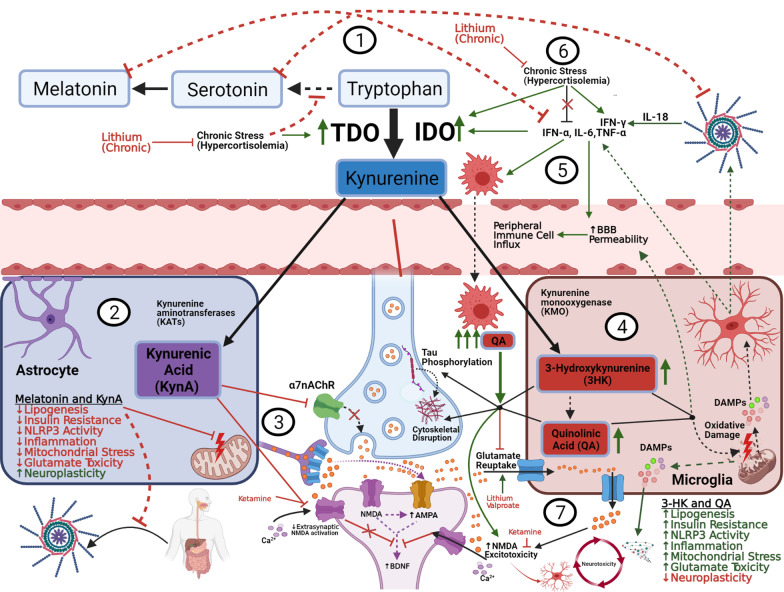
Fig. 4.
The Kynurenic pathway, glutamate, and neuroplasticity. (1) 95% of free, extracellular tryptophan is converted into KYN via IDO and TDO. BD, stress, and metabolic disease augment this conversion, decreasing free serotonin/melatonin with resultant increases in oxidative/inflammatory signaling. (2) Inside astrocytes, KYN is converted into KynA, which exerts a net neuroprotective effect by several mechanisms. (3) Through extra-synaptic NMDA-inhibition, ketamine shunts glutamate towards AMPA receptors and blocks QA influx, ultimately upregulating BDNF-mediated synaptic plasticity, in concert with an increase in KynA/QA ratio (NMDA receptor-kynurenic competition). (4) Inside microglia, KYN is converted to 3-HK and QA which exert various neurotoxic effects. (5) Proinflammatory cytokines increase QA production by increasing IDO activity and shunting tryptophan into microglia. This in turn amplifies the inflammatory signal, causing influx of peripheral immune cells, which exponentiate QA production and subsequent toxicity. (6) Under chronic stress (HPA overactivity), cortisol loses its feedback inhibition on pro-inflammatory signals, and continues to upregulate QA production though TDO/IDO induction and IFN-γ modulation, exacerbating the neurotoxic/inflammatory environment (discussed in Sect. 7). (7) Astrocytic processes form a “cradle” (left side) around synaptic connections and serve as the major site for glutamate reuptake, limiting excess glutamate spillover into extrasynaptic NMDA receptors. Astrocytes also exert negative feedback (not shown) to suppress microglial overactivation. Chronic inflammation, exacerbated by acute mood episodes (right side) disrupts the astrocytic cradle and impairs feedback mechanisms, resulting in extracellular glutamate accumulation and microglial overactivation. Activated microglia do engage in modest glutamate reuptake, but it is suppressed by QA and outpaced by their immune-stimulated release of various toxic metabolites (QA, ROS, Glutamate, cytokines, damaged organelles, etc.). Extrasynaptic NMDA activation suppresses BDNF expression, impairing synaptic homeostasis. Ultimately these signals result in a chronic reciprocal exacerbation of glutamate excitotoxicity, inflammation, and overall neurotoxicity (discussed in Sect. 8)