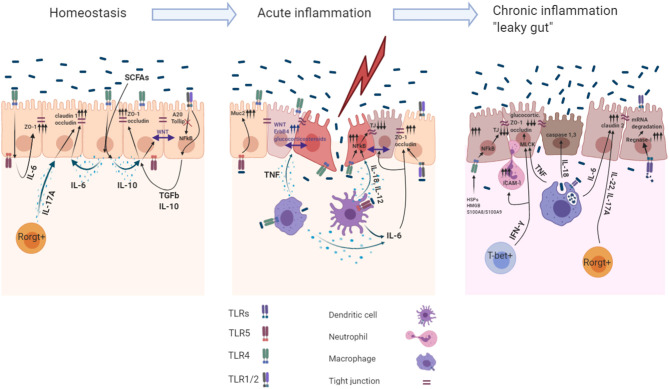
Figure 1.
Role of the TLR induced cytokines in acute and chronic intestinal inflammation. The intestinal mucosa is separated from the body immune environment by a single layer of the intestinal epithelial cells (IECs) that provides a physical and functional barrier. Beneath the IECs immune cells reside in the lamina propria, maintaining the intestinal tissue at the hyporesponsive state. Intestinal immune homeostasis: Constant recognition of microbiota by TLR4 and TLR1/2 leads to IL- 6, IL−10, TGF-β1 production within IECs. Autocrine recognition of these cytokines maintains IEC barrier integrity by promoting expression of the TJ proteins (ZO-1, claudin-1, occludin). Moreover, action of the IL-10 induce Wnt signaling within IECs, which maintains their proliferation. A20 and Tollip are the main inhibitors of the TLR1/2 signaling facilitating the avoidance of undesired response toward microbiota. Rorgt+ cells during homeostasis produce IL-17A to maintain constant production of claudin-1 and occluding within IECs. Acute inflammation: Sensing microbiota within the lamina propria induces production of pro-inflammatory cytokines and cytoprotective factors via NFkB dependent mechanism. Basolateral TLR5 mediated recognition of bacteria leads to MUC2 production in IECs. IL-6, TNF production by M?s and DCs during acute inflammation enables barrier repair program within IECs. TNF induced production of the glucocorticoids and ErbB4 receptor tyrosine kinase in IECs induce tissue repair functions and resolve late stages of the acute inflammation. Pro-inflammatory cytokines IL-18, IL-12 involved in IEC barrier dysfunction by downregulating TJ proteins (ZO-1, occludin). TLR1/2 basolateral recognition of bacteria promotes ZO-1/occluding expression in IECs. Chronic inflammation: Chronic TNF sensing by IECs reduces their ability to migrate toward crypts villi, modulates MLCK which decreases claudin-1, ZO-1, and ZO-2 expression and decreases glucocorticoids synthesis, which is indispensable for later inflammation resolution. IFN-γ activates the expression of ICAM-1 which resulted in increased IEC barrier permeability caused by neutrophil migration into subepithelial layers and paracellular space via modulation of MLCK. TLR4 dependent recognition of the HSPs, HMGB1 and S100A8/S100A9 by IECs leads to downregulation of the expression of ZO-1.