Abstract
Accumulating evidence indicates that ferroptosis is an iron-dependent form of regulated cell death. This type of iron-dependent programmed cell death is different from traditional forms of regulated cell death, such as apoptosis and autophagy. However, the role of ferroptosis in porcine oocyte maturation and the associated mechanism remain unclear. In the present research, we investigated the effects of ferric ammonium citrate (FAC), a specific ferroptosis inducer, on porcine oocyte meiotic maturation and quality and subsequent embryonic developmental competence. FAC treatment caused obvious accumulation of intracellular ferrous ions in porcine oocytes. At the end of the in vitro maturation (IVM) period, there was a significant decrease in the polar body (PB) extrusion rate and an increase in the percentage of abnormal oocytes in the FAC treatment groups, indicating that iron overload-induced ferroptosis may suppress the meiotic process during porcine oocyte maturation. We also found that after FAC treatment, the subsequent two-cell rate, four-cell rate and blastocyst formation rate were significantly decreased in porcine parthenogenetic activation (PA) embryos, indicating that iron overload-induced ferroptosis decreased porcine oocyte quality. Further analysis revealed that FAC treatment not only enhanced intracellular reactive oxygen species (ROS) generation, decreased intracellular free thiol levels and induced mitochondrial dysfunction but also triggered autophagy in porcine oocytes. Taken together, these findings suggest that iron overload-induced ferroptosis impairs porcine oocyte meiosis and decreases porcine oocyte quality, possibly by increasing oxidative stress, inducing mitochondrial dysfunction and triggering autophagy.
Keywords: iron overload, ferroptosis, porcine oocyte, oxidative stress, mitochondrial function
Introduction
With the development of livestock husbandry, an increasing number of assisted reproduction technologies, such as in vitro fertilization (IVF), somatic cell nuclear transfer (SCNT), and intracytoplasmic sperm injection (ICSI), have been widely used in the production of domestic animals. The implementation of these techniques needs to be accompanied by the use of high-quality in vitro- or in vivo-derived oocytes to be fully effective. Compared with in vivo-matured oocytes, in vitro-matured oocytes are easier to obtain. However, in vitro-matured oocytes are lower in quality and have a lower developmental potential than in vivo-matured oocytes. Oocyte in vitro maturation (IVM) is a complex process regulated by a large number of internal and external factors (Grupen, 2014). Any changes in this process lead to changes in oocyte quality, which affect the subsequent developmental capacity of preimplantation embryos (Koyama et al., 2014; Ahmed et al., 2017; Ferrer-Vaquer et al., 2019). Therefore, identifying the changes that occur in oocytes under stress conditions can help find potential solutions to reduce the corresponding negative effects.
Iron is a trace metal that is very important in mammalian physiological processes, such as DNA synthesis, energy generation, and oxygen transport, which rely on the existence of iron in variable and interconvertible oxidation states. However, dysregulation of iron homeostasis can lead to iron overload disorders, eventually resulting in excessive reactive oxygen species (ROS) generation and DNA damage and lipid peroxidation (Totsuka et al., 2019; Han et al., 2020; Tian et al., 2020). These events were defined as a form of programmed cell death called ferroptosis by Brent R. Stockwell’s team in 2012 (Dixon et al., 2012). Ferroptosis is a unique iron-dependent form of cell death (Xie et al., 2016; Tang et al., 2018) that is different from the traditional modes of cell death, such as necrosis (Pasparakis and Vandenabeele, 2015), autophagy (Glick et al., 2010), and apoptosis (Peña-Blanco and García-Sáez, 2018), in terms of cell morphology, biochemical characteristics and gene levels. Another characteristic of ferroptosis is the accumulation of ROS in cells (Sui et al., 2018). A large number of experiments have shown that ferroptosis occurs in neurodegenerative diseases (Abdalkader et al., 2018), infectious diseases (Matsushita et al., 2015), cancer (Chen et al., 2020), etc. Previous studies have found that under physiological and pathological conditions, a variety of hormonal (Belavgeni et al., 2019; Wang et al., 2019) and metabolic abnormalities (Stockwell et al., 2017; Wang et al., 2017) can trigger different types of cell death, including ferroptosis. Data from Zhang et al. (2020) showed that when the uterus and placenta of a female rat were dysfunctional, ferroptosis was triggered, and iron deposition occurred in the uterus. Furthermore, previous studies have also shown that the accumulation of iron and ferroptosis may occur in the early stage of follicular atresia (Zhang et al., 2018).
In the present research, a highly selective inducer of iron overload, ferric ammonium citrate (FAC), was used to establish an iron overload model in porcine oocytes. FAC, a trivalent iron salt, is absorbed in vivo by reducing trivalent iron to divalent ferrous iron (Cotticelli et al., 2019; Yao et al., 2021). It has been shown that FAC-induced intracellular iron overload causes ferroptosis (Fang et al., 2018). The aim of the present research was to determine whether iron overload-induced ferroptosis during IVM impairs meiotic maturation and developmental competence of porcine oocytes.
Materials and Methods
All chemicals used in this research were obtained from Sigma-Aldrich (St. Louis, MO, United States) unless otherwise noted.
Oocyte Collection and IVM
Porcine ovaries were obtained from a local slaughterhouse and transported to the laboratory in sterile 0.9% saline at 30–35°C. Cumulus-oocyte complexes (COCs) were obtained by aspirating 3∼8 mm antral follicles with a syringe. COCs with at least three or more layers of uniformly distributed cumulus cells were collected using Tyrode’s lactate-hydroxyethylpiperazine ethane sulfonic acid (HEPES) medium supplemented with 0.1% polyvinyl alcohol (PVA, w/v) and 0.05 g/L gentamycin under a stereomicroscope (S22-LGB, Nikon). The IVM medium consisted of tissue culture medium 199 (TCM-199, Invitrogen, Carlsbad, CA, United States) supplemented with 10% (v/v) porcine follicular fluid, 10 IU/mL follicle stimulating hormone (Ningbo No. 2 Hormone Factory, China), 10 IU/mL luteinizing hormone (Ningbo No. 2 Hormone Factory, China), 0.91 mM Na pyruvate, 10 ng/mL EGF, and 75 mg/mL kanamycin. The IVM medium was completely covered with mineral oil and cultured in an incubator containing 5% CO2 at 100% humidity at 38.5°C for 42 h.
For FAC treatment, FAC powder was dissolved in IVM medium at a concentration of 20 μM in the dark, and then 20 μM FAC solution was diluted in IVM medium to obtain 5 μM and 10 μM FAC solutions.
Parthenogenetic Activation (PA) and In vitro Culture (IVC)
Porcine oocyte PA was induced according to our previously described procedures (Qi et al., 2020). Briefly, cumulus cells were removed from COCs with cumulus cells expanded by 0.1% hyaluronidase at the end of the IVM period. Polar body (PB) extrusion of oocytes was examined under a stereomicroscope. The denuded oocytes were then subjected to electrical activation [300 mM mannitol containing 0.1 mM CaCl2, 0.05 mM MgSO4, 0.01% PVA (w/v), and 0.5 mM HEPES] at 110 V and 60 μs twice. After that, these oocytes were transferred to IVC medium [bicarbonate-buffered porcine zygote medium (PZM)-5 (Suzuki et al., 2007) comprising 4 mg/mL BSA] supplemented with 7.5 μg/mL cytochalasin B and cultured for 3 h to suppress extrusion of the pseudo-second PB. Next, the oocytes were thoroughly washed and cultured in IVC medium in four-well plates covered with mineral oil and cultured for 6.5 days at 38.5°C under 100% humidity and an atmosphere of 5% CO2 without changing the medium. Two-cell, four-cell and blastocyst formation rates were analyzed under a stereomicroscope at 24 h, 48 h, and 6.5 days. The two-cell, four-cell, and blastocyst formation rates were calculated by the number of examined embryos to the total embryos in each group.
Ferrous Ion Staining
Intracellular Fe2+ levels were examined at the end of the IVM period. The oocytes in each group were thoroughly washed in prewarmed PBS-PVA medium and assessed using the fluorescent probe Ferro Orange (Dojindo, F374) for 30 min. Images of the fluorescence signals were captured as TIFF files using a digital camera connected to a fluorescence microscope. The same procedures were followed for all groups of oocytes, including incubation, rinsing, mounting, and imaging. The fluorescence signal intensities of the oocytes in each group were analyzed via National Institutes of Health (NIH) ImageJ software (NIH, Bethesda, MD, United States).
Intracellular ROS Levels, Free Thiol Levels
Intracellular ROS levels and free thiol levels in oocytes were measured with an ROS detection kit (Thermo Fisher Scientific, C400) and free thiol level detection kit (Thermo Fisher Scientific, C12881). To determine intracellular ROS levels, oocytes were incubated for 15 min in PBS-PVA medium containing 10 μM 2′,7′-dichlorodihydrofluorescein diacetate. To determine intracellular free thiol levels, oocytes were incubated for 30 min in PBS-PVA medium containing 10 μM CMF2HC. Fluorescent signals were captured as a TIFF file using a digital camera connected to a fluorescence microscope. The same procedures were followed for all groups of oocytes, including incubation, rinsing, mounting, and imaging. The fluorescence signal intensities of the oocytes in each group were analyzed via NIH ImageJ software.
Mitochondrial Membrane Potential (MitoMP) Assessment
Mitochondrial membrane potential in oocytes was measured with a JC-1 MitoMP detection kit (Dojindo, MT09). Briefly, oocytes were incubated in PBS-PVA containing 2 μM JC-1 for 30 min. The MitoMP was calculated as a ratio of red florescence (J-aggregates; corresponding to activated mitochondria) to green fluorescence (J-monomers; corresponding to less active mitochondria). Images of the fluorescence signals were captured as TIFF files using a digital camera connected to a fluorescence microscope. The same procedures were followed for all groups of oocytes, including incubation, rinsing, mounting, and imaging. The fluorescence signal intensities of the oocytes in each group were analyzed via NIH ImageJ software.
Intracellular ATP Level Measurement
Intracellular ATP levels were measured using an ATP Detection Kit (Beyotime, S0027). Briefly, porcine oocytes from each group were collected and lysed with 200 μL of lysis buffer at the end of the IVM period. Next, the cell lysates were centrifuged at 12000 rpm at 4°C for 5 min, and the supernatant was taken for subsequent analysis. Then, 100 μL of ATP working solution and 20 μL of supernatant were added to 96-well opaque plates, which were analyzed with a luminometer (Tecan, Infinite M200 Pro).
Western Blotting Analysis
For Western blotting, 100 oocytes from each group were collected and fully lysed at 95°C in lysis buffer comprising 10% Tris–HCl, 40% DDH2O, 50% glycerol, 0.5 mM Tris–HCl, β-mercaptoethanol, and bromophenol blue. The protein samples were then loaded in a 10% polyacrylamide gel containing 0.1% SDS, and the separated proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Millipore). The PVDF membranes were blocked in 5% BSA at room temperature for 2 h and then incubated with primary antibodies against GAPDH (CST, #2118S), β-tubulin (Proteintech, 10094-1-AP), caspase-3 (Wanleibio, WL02117), Bcl-2 (Wanleibio, WL01556), Bax (Wanleibio, WL01637), GPX4 (BOSTER, BM5231), and LC-3 (CST, #11972S). After being washed with 1x TBST for 5 min each four times, the membranes were incubated at room temperature for 1 h with horseradish peroxidase-conjugated goat anti-rabbit IgG (Bioworld Technology, Inc., Louis Park, MN, United States, BS13278). The blots were visualized and analyzed by using a Tanon 5200 Image Analyzer (Tanon, Shanghai, China) and NIH ImageJ software, respectively.
Statistical Analysis
SPSS software version 11.0 (IBM, United States) was used to analyze all the data collected. Comparisons of data among groups were performed using one-way ANOVA or Student’s t-test. The results are presented as the mean ± standard error of mean (SEM) of the mean. Significant differences are indicated by different letters (p < 0.05).
Results
FAC Treatment Results in Intracellular Fe2+ Accumulation and Deterioration of Porcine Oocyte Quality
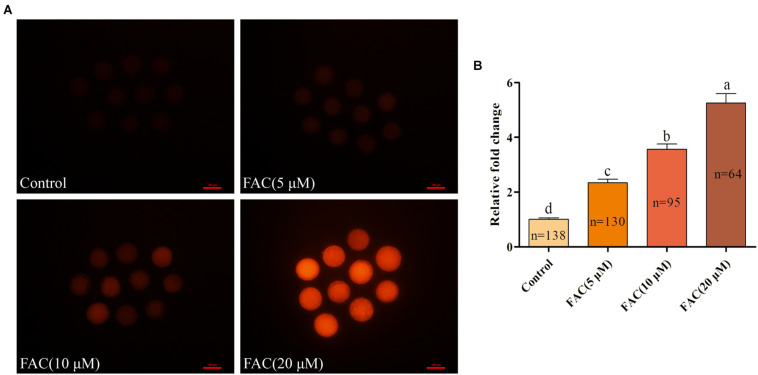
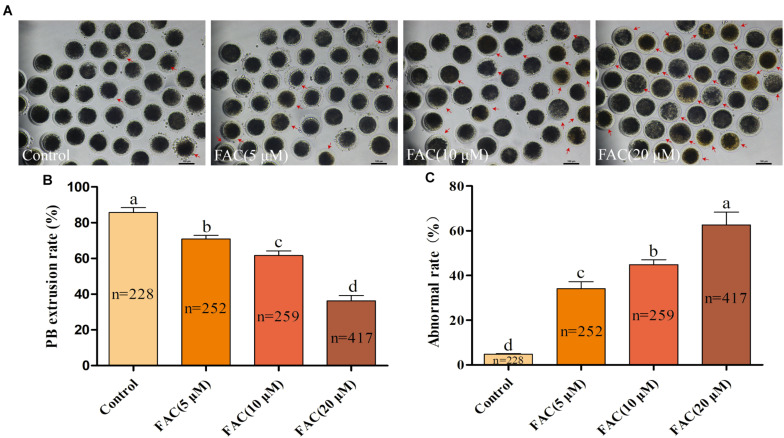
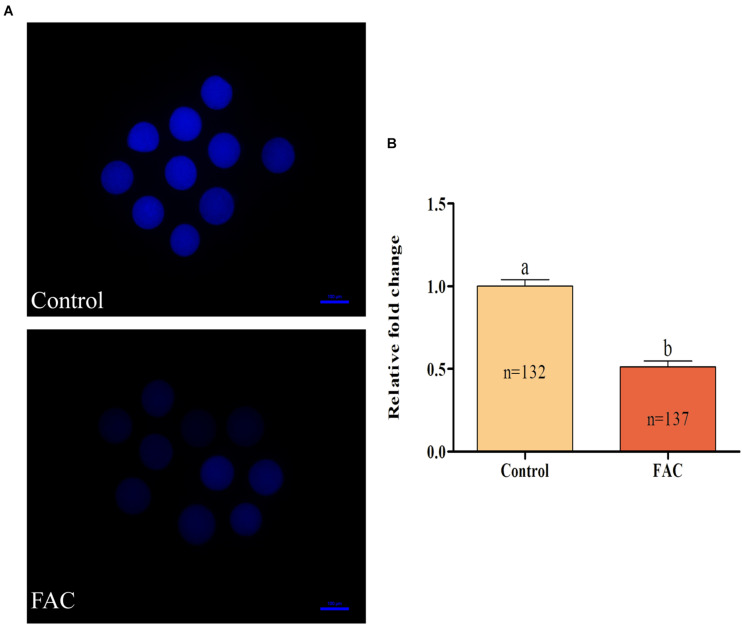
To investigate the potential involvement of ferroptosis in oocyte quality during IVM, porcine oocytes were treated with increasing concentrations of FAC (5 μM, 10 μM, and 20 μM), and intracellular Fe2+ levels, the rate of PB extrusion and the percentage of abnormal oocytes (Supplementary Figure 1) were analyzed. Analysis with the fluorescence probe FerroOrange revealed that the relative intracellular Fe2+ levels in oocytes increased in a concentration-dependent manner (Figures 1A,B). Further analysis revealed that FAC treatment decreased the rate of maturation (85.74 ± 2.69%, 70.86 ± 2.11%, 61.58 ± 2.66%, 36.27 ± 3.02%; p < <0.05) and increased the percentage of abnormal oocytes (4.80 ± 0.32%, 34.05 ± 3.19%, 44.84 ± 2.20%, 62.62 ± 5.75%; p < <0.05) in a dose-dependent manner (Figures 2A–C). In addition, FAC treatment impaired cumulus cell expansion capacity in porcine oocytes (Supplementary Figure 2). Western blotting analysis showed that the expression of the key ferroptosis factor GPX4 was upregulated, but there was no statistical significance in the expression of the apoptosis-related factors cleaved-caspase-3, BCL-2 and BAX in oocytes treated with FAC compared with oocytes in the control group (Supplementary Figures 3, 4). These results suggest that iron overload-induced ferroptosis has a direct negative effect on the porcine oocyte maturation process. According to our pre-experiment, 10 μM FAC was used for all subsequent experiments.
FIGURE 1.
Effects of FAC on intracellular Fe2+ accumulation in porcine oocytes during IVM. (A) Representative images of the fluorescent probe FerroOrange showing intracellular Fe2+ levels in porcine oocytes. Scale bar = 100 μm. (B) Quantification of the relative intracellular Fe2+ levels in porcine oocytes from the different FAC treatment groups. The number of oocytes examined from each experimental group is indicated by the bars. Statistically significant differences are represented by different letters (p < 0.05).
FIGURE 2.
FAC treatment impairs the porcine oocyte maturation process. (A) Representative images of porcine oocytes treated with different concentrations of FAC at the end of the IVM period are shown. Porcine oocytes with morphological abnormalities as examined by optical microscopy are indicated by arrows. Scale bar = 100 μm. (B) Oocyte PB extrusion rate in each experimental group. (C) Percentage of abnormal oocytes in each experimental group. The number of oocytes examined from each experimental group is indicated by the bars. Statistically significant differences are represented by different letters (p < 0.05).
Effects of FAC Treatment During IVM on Subsequent In vitro Embryo Development After PA
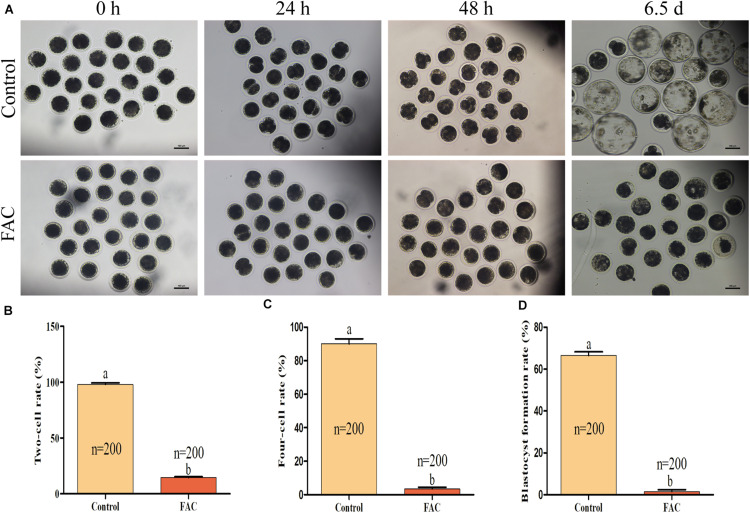
Since the quality of an oocyte directly affects its developmental potential, we next assessed whether FAC treatment during the IVM period decreased the developmental competence of porcine PA embryos. The results showed that FAC treatment had a negative effect on porcine embryo developmental competence (Figure 3A). The two-cell rates (Figure 3B; 98.00 ± 1.41% vs. 14.50 ± 0.96% at 24 h; p < 0.05), four-cell rates (Figure 3C; 90.00 ± 3.16% vs. 3.50 ± 0.96% at 48 h; p < 0.05), and blastocyst formation rates (Figure 3D; 66.50 ± 1.71% vs. 1.50 ± 0.96% on day 6.5; p < 0.05) of the PA embryos generated from mature oocytes from the FAC-treated group were significantly lower than those of PA embryos generated from mature oocytes from the control group.
FIGURE 3.
Developmental competence of porcine oocytes after FAC treatment. (A) Development of PA embryos from the control and FAC treatment groups at different time points. Scale bar = 100 μm. (B) Two-cell rate of PA embryos from the control and FAC treatment groups. (C) Four-cell rate of PA embryos from the control and FAC treatment groups. (D) Blastocyst formation rate of PA embryos from the control and FAC treatment groups. The number of embryos examined from each experimental group is indicated by the bars. Statistically significant differences are represented by different letters (p < 0.05).
Effects of FAC Treatment During IVM on the Oxidative Resistance of Porcine Oocytes
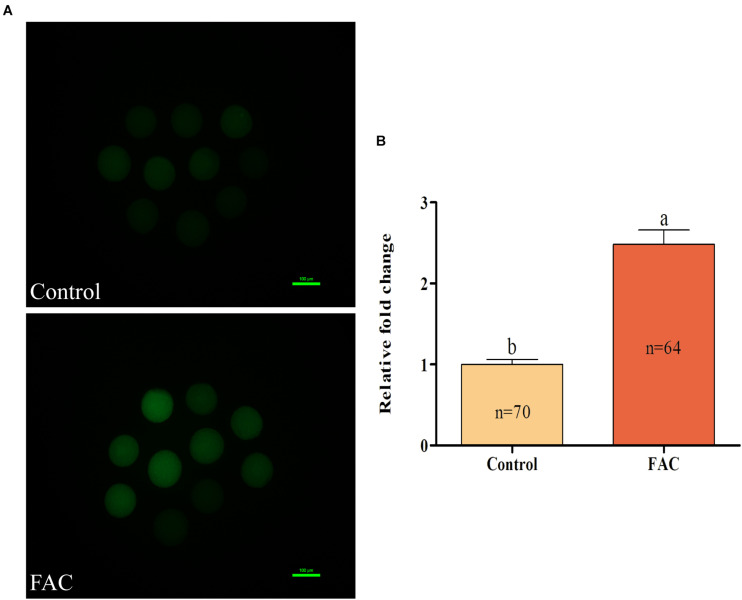
To analyze the mechanism through which FAC-induced ferroptosis affects porcine oocyte maturation, intracellular ROS and free thiol levels in FAC-treated oocytes were measured. Intracellular ROS levels were measured by assessing DCFH fluorescence (Figure 4A). Quantitative analysis showed that the relative intracellular ROS levels in porcine oocytes were significantly increased in the FAC treatment group compared with the control group (Figure 4B; p < 0.05). Next, the intracellular free thiol levels in porcine oocytes were measured (Figure 5A). As shown in Figure 5B, quantitative analysis showed that the relative intracellular free thiol levels were significantly lower in the FAC treatment group than in the control group (p < 0.05), suggesting that iron overload-induced ferroptosis can lead to oxidative stress and decrease the oxidative resistance of porcine oocytes.
FIGURE 4.
Effects of FAC treatment on intracellular ROS generation in porcine oocytes during IVM. (A) Representative fluorescence images showing intracellular ROS levels in porcine oocytes. Scale bar = 100 μm. (B) Quantification of relative intracellular ROS levels in porcine oocytes from the control and FAC treatment groups. The number of oocytes examined from each experimental group is indicated by the bars. Statistically significant differences are represented by different letters (p < 0.05).
FIGURE 5.
Effects of FAC treatment on intracellular free thiol levels in porcine oocytes during IVM. (A) Representative fluorescence images showing intracellular free thiol levels in porcine oocytes. Scale bar = 100 μm. (B) Quantification of relative intracellular free thiol levels in porcine oocytes from the control and FAC treatment groups. The number of oocytes examined from each experimental group is indicated by the bars. Statistically significant differences are represented by different letters (p < 0.05).
Effects of FAC Treatment During IVM on Mitochondrial Function in Porcine Oocytes
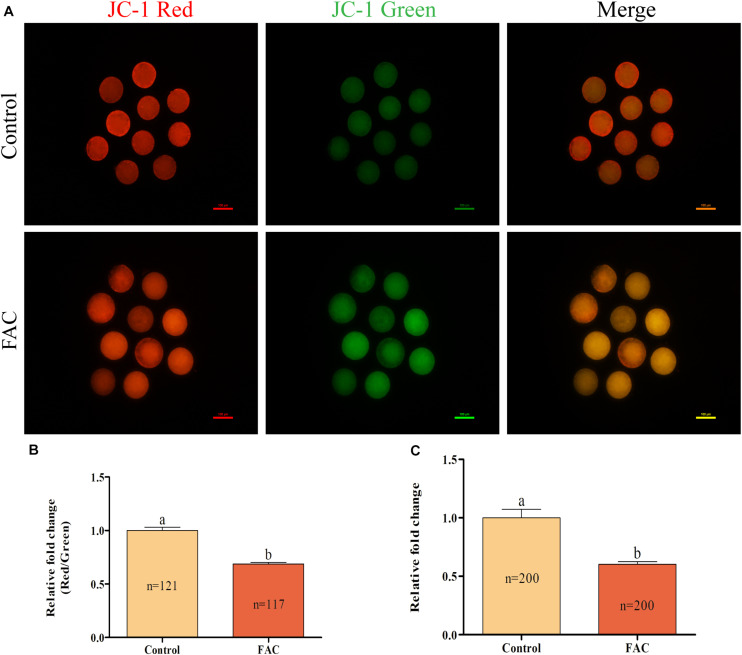
As the source of energy for cells, mitochondria play a vital role in the oocyte maturation process. Therefore, the intracellular MitoMP and ATP levels in porcine oocytes were analyzed. The intracellular MitoMP of porcine oocytes was evaluated using JC-1 fluorescent dye (Figure 6A). Quantitative analysis showed that the relative intracellular MitoMP of porcine oocytes was decreased in the FAC treatment group compared with the control group (Figure 6B; p < 0.05). Further analysis showed that the relative intracellular ATP levels in porcine oocytes were significantly lower in the FAC treatment group than in the control group (Figure 6C; p < 0.05). These results indicate that iron overload-induced ferroptosis can impair mitochondrial function in porcine oocytes.
FIGURE 6.
Effects of FAC treatment on mitochondrial function in porcine oocytes during IVM. (A) Representative fluorescence images of JC-1-stained porcine oocytes. Scale bar = 100 μm. (B) Quantification of the relative JC-1 fluorescence intensity in porcine oocytes from the control and FAC treatment groups. (C) Quantification of relative intracellular ATP levels in porcine oocytes from the control and FAC treatment groups. The number of oocytes examined from each experimental group is indicated by the bars. Statistically significant differences are represented by different letters (p < 0.05).
Effects of FAC Treatment During IVM on Autophagy in Porcine Oocytes
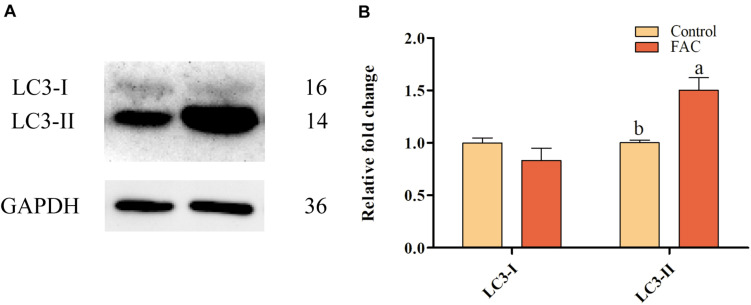
To evaluate whether FAC-induced ferroptosis can induce autophagy in porcine oocytes, the protein expression of LC3, which is associated with autophagy, in porcine oocytes was analyzed after FAC treatment. Western blotting analysis showed that LC3-II protein expression was upregulated in oocytes treated with FAC compared with oocytes in the control group (Figure 7). This result indicates that iron overload is related to the induction of autophagy in porcine oocytes.
FIGURE 7.
Effects of FAC treatment on autophagy in porcine oocytes during IVM. (A) Western blotting analysis of LC3 expression in porcine oocytes. (B) Quantitative LC3-I and LC3-II levels in porcine oocytes. Statistically significant differences are represented by different letters (p < 0.05).
Discussion
The present research suggested that iron overload disorders induced by FAC decreased porcine oocyte quality by increasing intracellular ROS generation, decreasing intracellular free thiol levels, and inducing mitochondrial dysfunction during IVM. Importantly, subsequent embryonic developmental potential was markedly decreased following iron overload during IVM of porcine oocytes. These results suggest that dysregulation of iron homeostasis decreases porcine oocyte quality and subsequent embryonic developmental competence.
Iron overload induces ferroptosis characterized by phospholipid peroxidation of plasma membranes caused by ROS generated during iron-mediated Fenton reactions (Dixon et al., 2012). A previous study showed that iron overload induces ferroptosis in cells and a loss of antioxidant defense (Chen et al., 2021). Porcine oocytes have relatively higher intracellular lipid levels than oocytes of other species, making them highly sensitive to ROS-induced impairments (Gajda, 2009). A previous study suggested that excessive intracellular ROS accumulation can induce cell cycle arrest and apoptosis in oocytes (Tripathi et al., 2009; Tiwari et al., 2017). It was found that oxidative stress can lead to a decrease in oocyte quality and reduce subsequent embryonic developmental competence (Yu et al., 2019; Zhou et al., 2019). In the present study, we found that FAC-induced iron overload led to intracellular ROS generation in porcine oocytes. To further evaluate the underlying process and mechanism through which FAC-induced iron overload decreases the quality and developmental potential of porcine oocytes, we examined intracellular free thiol levels. The levels of intracellular free thiols are regarded as important indicators of cytoplasmic maturation of oocytes at the end of the IVM period (Liang et al., 2018; Zhang et al., 2019). Several studies have shown that oocytes with higher intracellular ROS levels have lower intracellular free thiol levels and insufficient embryonic developmental potential (Nabenishi et al., 2012; Liang et al., 2017b; Li and Zhao, 2019). In the present study, FAC-induced iron overload during IVM decreased intracellular free thiol levels in the cytoplasm. These results are consistent with our hypothesis that FAC-induced iron overload decreases porcine oocyte quality by consuming intracellular free thiols and inducing the accumulation of intracellular ROS.
Mitochondria are a site of energy metabolism and are involved in cell apoptosis and death. Several studies have shown that mitochondrial function influences oocyte developmental potential and is associated with subsequent embryonic development, such as that of PA, IVF, and SCNT embryos (Liang et al., 2018; An et al., 2019; Hu et al., 2020; Nie et al., 2020). Recent research has suggested that ferroptosis can lead to mitochondrial dysfunction, including loss of the MitoMP, enhanced mitochondrial fragmentation, and reduced mitochondrial respiration, in neuronal HT22 cells and mouse embryonic fibroblasts (Jelinek et al., 2018). In addition, in vivo studies have suggested that excessive iron accumulation induces ferroptosis, not only exacerbating mitochondrial dysfunction but also increasing intracellular ROS and malondialdehyde levels (Kumfu et al., 2016, 2018; Wongjaikam et al., 2016, 2017; Khamseekaew et al., 2017; Sumneang et al., 2020). Abdalkader et al. (2018) also found that the characteristics of many neurodegenerative diseases are similar to those of ferroptosis-associated conditions, such as iron accumulation disorders and mitochondrial dysfunction. The MitoMP is commonly used as an indicator of mitochondrial function in oocytes (Liang et al., 2018) and is the driving force behind intracellular ATP synthesis (Dimroth et al., 2000). There is increasing evidence that oocytes with a higher MitoMP have better developmental potential (Liang et al., 2017a; An et al., 2019; Nie et al., 2020; Niu et al., 2020). Previous studies have suggested that iron overload could induce apoptosis through mitochondrial dysfunction, which increased mitochondrial oxidative stress and activated the caspase-dependent apoptotic pathway (Khamseekaew et al., 2017; Kumfu et al., 2018). Therefore, we analyzed intracellular MitoMP and ATP production in porcine oocytes after FAC treatment. Mitochondrial functional assays revealed that intracellular MitoMP and ATP production exhibited significant decreasing trends. These changes may account for the decrease in the quality of porcine oocytes after FAC-induced iron overload as well as the reduction in oocyte developmental potential. A similar study of mouse spermatozoa revealed that iron overload significantly decreases motility, viability, MitoMP, and GPX activity and increases the generation of ROS (Mojica-Villegas et al., 2014). Further studies, including studies on mitochondrial dysfunction and transmission electron microscopy results of mitochondria, are needed to further investigate the mechanism by which iron overload decreases the developmental competence of oocytes in pigs.
Autophagy is an intracellular process of self-degradation that occurs in abnormal physiological processes. LC3 is an autophagosome-labeling protein. LC3I exists in two forms: LC3I is lipidated and ubiquitylated into LC3II, which is ultimately targeted to the autophagosome or its precursor (Kabeya et al., 2000). It has been suggested that the modification of LC3I to LC3II is a sign of autophagy (Kabeya et al., 2004). In the present research, FAC-induced iron overload upregulated the expression of LC3II in porcine oocytes. This result was consistent with previous studies showing that iron overload-induced ferroptosis triggers autophagy in L6 skeletal muscle cells (Jahng et al., 2019) and murine preosteoblast cells (Cen et al., 2018). Thus, iron overload-induced ferroptosis might trigger autophagy to affect porcine oocyte meiotic maturation and block further development.
Conclusion
Taken together, the present research demonstrated that iron overload-induced ferroptosis might decrease porcine oocyte quality by inducing intracellular ROS generation and decreasing intracellular free thiol levels and mitochondrial dysfunction. These findings provide novel insights into the mechanisms underlying iron overload-induced ferroptosis in oocytes. In the future, in vivo experiments should be carried out to confirm the effect of iron overload-induced ferroptosis on porcine oocyte maturation and reduce the limitations of in vitro-matured oocyte tests.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The present research followed the Care and Use of Laboratory Animals prepared by the Institutional Animal Care and Use Committee of Jilin University, China.
Author Contributions
WH, YaZ, BS, and SL participated in the research design and wrote the article. WH, YaZ, DW, TY, and JQ participated in the experiment and data analysis. WH, YoZ, SL, HJ, and JZ participated in revising the article. All authors approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This research was supported by the National Natural Science Foundation of China (31802060) and Jilin Scientific and Technological Development Program of China (SXGJSF2017-6).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.673291/full#supplementary-material
References
- Abdalkader M., Lampinen R., Kanninen K. M., Malm T. M., Liddell J. R. (2018). Targeting Nrf2 to Suppress Ferroptosis and Mitochondrial Dysfunction in Neurodegeneration. Front. Neurosci. 12:466. 10.3389/fnins.2018.00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed J. A., Nashiruddullah N., Dutta D., Biswas R. K., Borah P. (2017). Cumulus cell expansion and ultrastructural changes in in vitro matured bovine oocytes under heat stress. Iran. J. Vet. Res. 18 203–207. [PMC free article] [PubMed] [Google Scholar]
- An Q., Peng W., Cheng Y., Lu Z., Zhou C., Zhang Y., et al. (2019). Melatonin supplementation during in vitro maturation of oocyte enhances subsequent development of bovine cloned embryos. J. Cell. Physiol. 234 17370–17381. 10.1002/jcp.28357 [DOI] [PubMed] [Google Scholar]
- Belavgeni A., Bornstein S. R., von Mässenhausen A., Tonnus W., Stumpf J., Meyer C., et al. (2019). Exquisite sensitivity of adrenocortical carcinomas to induction of ferroptosis. Proc. Natl. Acad. Sci. U. S. A. 116 22269–22274. 10.1073/pnas.1912700116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen W. J., Feng Y., Li S. S., Huang L. W., Zhang T., Zhang W., et al. (2018). Iron overload induces G1 phase arrest and autophagy in murine preosteoblast cells. J. Cell. Physiol. 233 6779–6789. 10.1002/jcp.26405 [DOI] [PubMed] [Google Scholar]
- Chen G., Guo G., Zhou X., Chen H. (2020). Potential mechanism of ferroptosis in pancreatic cancer. Oncol. Lett. 19 579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Comish P. B., Tang D., Kang R. (2021). Characteristics and Biomarkers of Ferroptosis. Front. Cell Dev. Biol. 9:637162. 10.3389/fcell.2021.637162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotticelli M. G., Xia S., Lin D., Lee T., Terrab L., Wipf P., et al. (2019). Ferroptosis as a Novel Therapeutic Target for Friedreich’s Ataxia. J. Pharmacol. Exp. Ther. 369 47–54. [DOI] [PubMed] [Google Scholar]
- Dimroth P., Kaim G., Matthey U. (2000). Crucial role of the membrane potential for ATP synthesis by F(1)F(o) ATP synthases. J. Exp. Biol. 203 51–59. 10.1242/jeb.203.1.51 [DOI] [PubMed] [Google Scholar]
- Dixon S. J., Lemberg K. M., Lamprecht M. R., Skouta R., Zaitsev E. M., Gleason C. E., et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149 1060–1072. 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S., Yu X., Ding H., Han J., Feng J. (2018). Effects of intracellular iron overload on cell death and identification of potent cell death inhibitors. Biochem. Biophys. Res. Commun. 503 297–303. 10.1016/j.bbrc.2018.06.019 [DOI] [PubMed] [Google Scholar]
- Ferrer-Vaquer A., Barragán M., Rodríguez A., Vassena R. (2019). Altered cytoplasmic maturation in rescued in vitro matured oocytes. Hum. Reprod. 34 1095–1105. 10.1093/humrep/dez052 [DOI] [PubMed] [Google Scholar]
- Gajda B. (2009). Factors and methods of pig oocyte and embryo quality improvement and their application in reproductive biotechnology. Reprod. Biol. 9 97–112. 10.1016/s1642-431x(12)60020-5 [DOI] [PubMed] [Google Scholar]
- Glick D., Barth S., Macleod K. F. (2010). Autophagy: cellular and molecular mechanisms. J. Pathol. 221 3–12. 10.1002/path.2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupen C. G. (2014). The evolution of porcine embryo in vitro production. Theriogenology 81 24–37. 10.1016/j.theriogenology.2013.09.022 [DOI] [PubMed] [Google Scholar]
- Han Z., Xu Z., Chen L., Ye D., Yu Y., Zhang Y., et al. (2020). Iron overload inhibits self-renewal of human pluripotent stem cells via DNA damage and generation of reactive oxygen species. FEBS Open Bio 10 726–733. 10.1002/2211-5463.12811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. Y., Li X. X., Diao Y. F., Qi J. J., Wang D. L., Zhang J. B., et al. (2020). Asiatic acid protects oocytes against in vitro aging-induced deterioration and improves subsequent embryonic development in pigs. Aging 13 3353–3367. 10.18632/aging.202184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng J. W. S., Alsaadi R. M., Palanivel R., Song E., Hipolito V. E. B., Sung H. K., et al. (2019). Iron overload inhibits late stage autophagic flux leading to insulin resistance. EMBO Rep. 20:e47911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek A., Heyder L., Daude M., Plessner M., Krippner S., Grosse R., et al. (2018). Mitochondrial rescue prevents glutathione peroxidase-dependent ferroptosis. Free Radic. Biol. Med. 117 45–57. 10.1016/j.freeradbiomed.2018.01.019 [DOI] [PubMed] [Google Scholar]
- Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., et al. (2000). LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J. 19 5720–5728. 10.1093/emboj/19.21.5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y., Mizushima N., Yamamoto A., Oshitani-Okamoto S., Ohsumi Y., Yoshimori T. (2004). LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J. Cell Sci. 117 2805–2812. 10.1242/jcs.01131 [DOI] [PubMed] [Google Scholar]
- Khamseekaew J., Kumfu S., Wongjaikam S., Kerdphoo S., Jaiwongkam T., Srichairatanakool S., et al. (2017). Effects of iron overload, an iron chelator and a T-Type calcium channel blocker on cardiac mitochondrial biogenesis and mitochondrial dynamics in thalassemic mice. Eur. J. Pharmacol. 799 118–127. 10.1016/j.ejphar.2017.02.015 [DOI] [PubMed] [Google Scholar]
- Koyama K., Kang S. S., Huang W., Yanagawa Y., Takahashi Y., Nagano M. (2014). Aging-related changes in in vitro-matured bovine oocytes: oxidative stress, mitochondrial activity and ATP content after nuclear maturation. J. Reprod. Dev. 60 136–142. 10.1262/jrd.2013-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfu S., Chattipakorn S. C., Fucharoen S., Chattipakorn N. (2016). Dual T-type and L-type calcium channel blocker exerts beneficial effects in attenuating cardiovascular dysfunction in iron-overloaded thalassaemic mice. Exp. Physiol. 101 521–539. 10.1113/ep085517 [DOI] [PubMed] [Google Scholar]
- Kumfu S., Khamseekaew J., Palee S., Srichairatanakool S., Fucharoen S., Chattipakorn S. C., et al. (2018). A combination of an iron chelator with an antioxidant exerts greater efficacy on cardioprotection than monotherapy in iron-overload thalassemic mice. Free Radic. Res. 52 70–79. 10.1080/10715762.2017.1414208 [DOI] [PubMed] [Google Scholar]
- Li Q., Zhao Z. (2019). Influence of N-acetyl-L-cysteine against bisphenol a on the maturation of mouse oocytes and embryo development: in vitro study. BMC Pharmacol. Toxicol. 20:43. 10.1186/s40360-019-0323-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S., Guo J., Choi J. W., Kim N. H., Cui X. S. (2017a). Effect and possible mechanisms of melatonin treatment on the quality and developmental potential of aged bovine oocytes. Reprod. Fertil. Dev. 29 1821–1831. 10.1071/rd16223 [DOI] [PubMed] [Google Scholar]
- Liang S., Nie Z. W., Zhao M., Niu Y. J., Shin K. T., Cui X. S. (2017b). Sodium fluoride exposure exerts toxic effects on porcine oocyte maturation. Sci Rep. 7:17082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S., Guo J., Jin Y. X., Yuan B., Zhang J. B., Kim N. H. (2018). C-Phycocyanin supplementation during in vitro maturation enhances pre-implantation developmental competence of parthenogenetic and cloned embryos in pigs. Theriogenology 106 69–78. 10.1016/j.theriogenology.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Matsushita M., Freigang S., Schneider C., Conrad M., Bornkamm G. W., Kopf M. (2015). T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 212 555–568. 10.1084/jem.20140857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojica-Villegas M. A., Izquierdo-Vega J. A., Chamorro-Cevallos G., ánchez-Gutiérrez M. S. (2014). Protective effect of resveratrol on biomarkers of oxidative stress induced by iron/ascorbate in mouse spermatozoa. Nutrients 6 489–503. 10.3390/nu6020489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabenishi H., Ohta H., Nishimoto T., Morita T., Ashizawa K., Tsuzuki Y. (2012). The effects of cysteine addition during in vitro maturation on the developmental competence, ROS, GSH and apoptosis level of bovine oocytes exposed to heat stress. Zygote 20 249–259. 10.1017/s0967199411000220 [DOI] [PubMed] [Google Scholar]
- Nie J., Yan K., Sui L., Zhang H., Zhang H., Yang X., et al. (2020). Mogroside V improves porcine oocyte in vitro maturation and subsequent embryonic development. Theriogenology 141 35–40. 10.1016/j.theriogenology.2019.09.010 [DOI] [PubMed] [Google Scholar]
- Niu Y. J., Zhou W., Nie Z. W., Shin K. T., Cui X. S. (2020). Melatonin enhances mitochondrial biogenesis and protects against rotenone-induced mitochondrial deficiency in early porcine embryos. J. Pineal Res. 68:e12627. [DOI] [PubMed] [Google Scholar]
- Pasparakis M., Vandenabeele P. (2015). Necroptosis and its role in inflammation. Nature 517 311–320. 10.1038/nature14191 [DOI] [PubMed] [Google Scholar]
- Peña-Blanco A., García-Sáez A. J. (2018). Bax, Bak and beyond - mitochondrial performance in apoptosis. FEBS J. 285 416–431. 10.1111/febs.14186 [DOI] [PubMed] [Google Scholar]
- Qi J. J., Li X. X., Diao Y. F., Liu P. L., Wang D. L., Bai C. Y., et al. (2020). Asiatic acid supplementation during the in vitro culture period improves early embryonic development of porcine embryos produced by parthenogenetic activation, somatic cell nuclear transfer and in vitro fertilization. Theriogenology 142 26–33. 10.1016/j.theriogenology.2019.09.027 [DOI] [PubMed] [Google Scholar]
- Stockwell B. R., Friedmann Angeli J. P., Bayir H., Bush A. I., Conrad M., Dixon S. J., et al. (2017). Ferroptosis: a Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171 273–285. 10.1016/j.cell.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X., Zhang R., Liu S., Duan T., Zhai L., Zhang M., et al. (2018). RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front. Pharmacol. 9:1371. 10.3389/fphar.2018.01371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumneang N., Siri-Angkul N., Kumfu S., Chattipakorn S. C., Chattipakorn N. (2020). The effects of iron overload on mitochondrial function, mitochondrial dynamics, and ferroptosis in cardiomyocytes. Arch. Biochem. Biophys. 680:108241. 10.1016/j.abb.2019.108241 [DOI] [PubMed] [Google Scholar]
- Suzuki C., Yoshioka K., Sakatani M., Takahashi M. (2007). Glutamine and hypotaurine improves intracellular oxidative status and in vitro development of porcine preimplantation embryos. Zygote 15 317–324. 10.1017/s0967199407004273 [DOI] [PubMed] [Google Scholar]
- Tang M., Chen Z., Wu D., Chen L. (2018). Ferritinophagy/ferroptosis: iron-related newcomers in human diseases. J. Cell. Physiol. 233 9179–9190. 10.1002/jcp.26954 [DOI] [PubMed] [Google Scholar]
- Tian Q., Qin B., Gu Y., Zhou L., Chen S., Zhang S., et al. (2020). ROS-Mediated Necroptosis Is Involved in Iron Overload-Induced Osteoblastic Cell Death. Oxid. Med. Cell. Longev. 2020:1295382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari M., Prasad S., Shrivastav T. G., Chaube S. K. (2017). Calcium Signaling During Meiotic Cell Cycle Regulation and Apoptosis in Mammalian Oocytes. J. Cell. Physiol. 232 976–981. 10.1002/jcp.25670 [DOI] [PubMed] [Google Scholar]
- Totsuka K., Ueta T., Uchida T., Roggia M. F., Nakagawa S., Vavvas D. G., et al. (2019). Oxidative stress induces ferroptotic cell death in retinal pigment epithelial cells. Exp. Eye Res. 181 316–324. 10.1016/j.exer.2018.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A., Khatun S., Pandey A. N., Mishra S. K., Chaube R., Shrivastav T. G., et al. (2009). Intracellular levels of hydrogen peroxide and nitric oxide in oocytes at various stages of meiotic cell cycle and apoptosis. Free Radic. Res. 43 287–294. 10.1080/10715760802695985 [DOI] [PubMed] [Google Scholar]
- Wang H., An P., Xie E., Wu Q., Fang X., Gao H., et al. (2017). Characterization of ferroptosis in murine models of hemochromatosis. Hepatology 66 449–465. 10.1002/hep.29117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang Z. B., Luo C., Mao X. Y., Li X., Yin J. Y., et al. (2019). The Prospective Value of Dopamine Receptors on Bio-Behavior of Tumor. J. Cancer 10 1622–1632. 10.7150/jca.27780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongjaikam S., Kumfu S., Khamseekaew J., Chattipakorn S. C., Chattipakorn N. (2017). Restoring the impaired cardiac calcium homeostasis and cardiac function in iron overload rats by the combined deferiprone and N-acetyl cysteine. Sci. Rep. 7:44460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongjaikam S., Kumfu S., Khamseekaew J., Sripetchwandee J., Srichairatanakool S., Fucharoen S., et al. (2016). Combined Iron Chelator and Antioxidant Exerted Greater Efficacy on Cardioprotection Than Monotherapy in Iron-Overloaded Rats. PLoS One 11:e0159414. 10.1371/journal.pone.0159414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Hou W., Song X., Yu Y., Huang J., Sun X., et al. (2016). Ferroptosis: process and function. Cell Death Differ. 23 369–379. 10.1038/cdd.2015.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Sun K., Yu S., Luo J., Guo J., Lin J., et al. (2021). Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J. Orthop. Translat. 27 33–43. 10.1016/j.jot.2020.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Zhao Y., Feng Y., Zhang H., Li L., Shen W., et al. (2019). β-carotene improves oocyte development and maturation under oxidative stress in vitro. In Vitro Cell. Dev. Biol. Anim. 55 548–558. 10.1007/s11626-019-00373-0 [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu Y., Yao W., Li Q., Liu H., Pan Z. (2018). Initiation of follicular atresia: gene networks during early atresia in pig ovaries. Reproduction 156 23–33. 10.1530/rep-18-0058 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Guo J., Nie X. W., Li Z. Y., Wang Y. M., Liang S., et al. (2019). Rosmarinic acid treatment during porcine oocyte maturation attenuates oxidative stress and improves subsequent embryo development in vitro. PeerJ 7:e6930. 10.7717/peerj.6930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Hu M., Jia W., Liu G., Zhang J., Wang B., et al. (2020). Hyperandrogenism and insulin resistance modulate gravid uterine and placental ferroptosis in PCOS-like rats. J. Endocrinol. 246 247–263. 10.1530/joe-20-0155 [DOI] [PubMed] [Google Scholar]
- Zhou C., Zhang X., Chen Y., Liu X., Sun Y., Xiong B. (2019). Glutathione alleviates the cadmium exposure-caused porcine oocyte meiotic defects via eliminating the excessive ROS. Environ. Pollut. 255:113194. 10.1016/j.envpol.2019.113194 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.