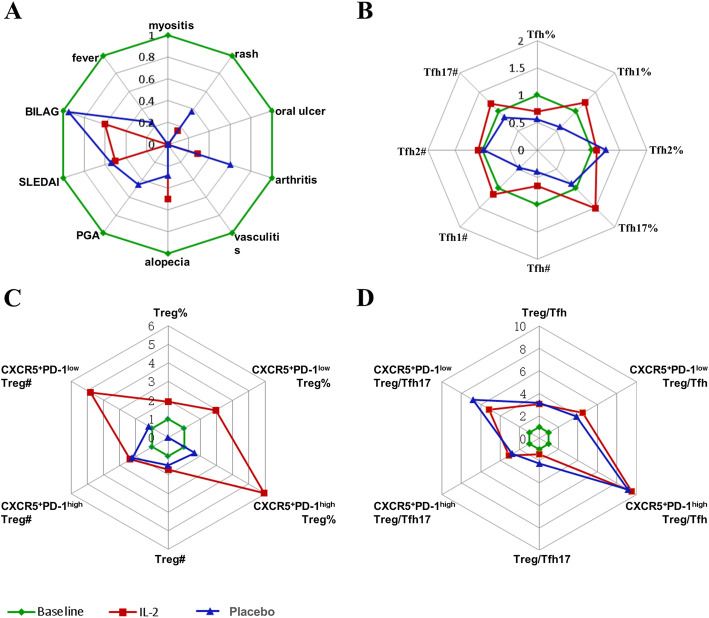
Fig. 4.
The response of clinical and immune cells to low-dose IL-2 (n=30) and placebo (n=30) therapy in SLE. A Relative change of disease activity value and patient number. B Relative change of ratios at baseline and week 12. C Relative change of Treg subsets at baseline and week 12. D Relative change of Tfh subsets at baseline and week 12. BILAG, British Isles Lupus Assessment Group. SLEDAI, Safety of Estrogens in Lupus Erythematosus National Assessment version of the SLE Disease Activity Index. SRI-4, SLE Responder Index-4. PGA, physician’s global assessment. All data here were normalized to baseline values to show relative changes. In detail, all baseline data and data after therapy were divided by the value of baseline. #, absolute number, cells/μl. %, proportion of cells in lymphocyte