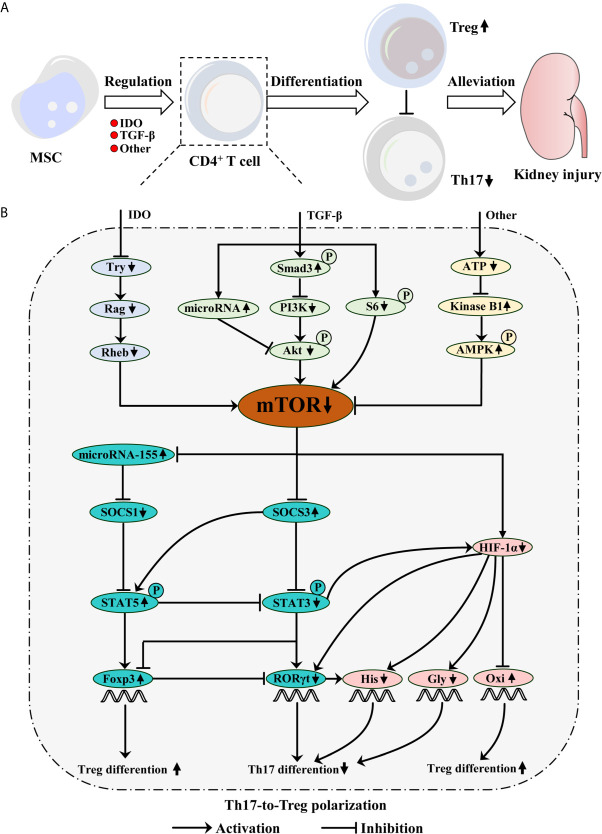
Figure 1.
Mesenchymal stem cell (MSC) protects kidney injury via mTOR-mediated Th17-to-Treg polarization. (A) MSC inhibits mTOR signaling during CD4+ T cell differentiation via IDO and TGF-β secretions as well as other mechanisms, which restrains Th17 differentiation, but promotes Treg generation. Consequently, MSC-mediated Th17-to-Treg polarization alleviates kidney injury. (B) TGF-β phosphorylates Smad3, which inhibits PI3K/Akt/mTOR pathway. TGF-β also inhibits S6-mediated mTOR signaling. Moreover, TGF-β promotes microRNA expression, which can inhibit Akt/mTOR pathway. IDO can deplete tryptophan (Try), which results in mTOR inhibition mediated by the Rag and following Rheb. Additionally, MSC-mediated low ATP concentration increases kinase B1, subsequently phosphorylates AMPK, ultimately inhibits mTOR signaling. However, mTOR signaling can inhibit microRNA-155 and SOCS3 expression, but promote HIF-1α generation. Briefly, microRNA-155 inhibits SOCS1 expression, which can restrain STAT5 phosphorylation. The phosphorylation of STAT5 not only restrains STAT3 phosphorylation, but also upregulates Foxp3 expression, which promotes Treg differentiation. SOCS3 can promote STAT5 phosphorylation, but inhibit STAT3-mediated HIF-1α and RORγt expression, which ultimately reduces Th17 differentiation. Moreover, STAT3 possesses the ability to inhibit Foxp3 expression. HIF-1α can promote RORγt expression and increase histone acetylation (His) with RORγt collaboration, which possesses the ability to induce Th17 differentiation. Meanwhile, HIF-1α inhibition can promote Treg but not Th17 differentiation by mediating glycolysis (Gly) switching into the oxidative phosphorylation (Oxi).