TABLE 3.
Identification of in vivo metabolites of hyzetimibe.
| Component | Structure proposal | MW (Da) | Retention time (min) | m/z | m/z14C | Typical MS fragment | Matrix |
|---|---|---|---|---|---|---|---|
| Hyzetimibe |

|
421 | 37.1 | 422.1553 | 422.1605 | 265–293–404 | P, U, F |
| M1 |

|
597 | 27.9–28.1 | 598.1863 | 600.1914 | 422–404–293–265 | P, U |
| M2 |

|
597 | 28.6–28.9 | 598.1864 | 600.1917 | 422–404 | U |
| M3 |

|
613 | 27.1–27.4 | 614.1818 | 616.3768 | 438–420–281 | P, U |
| M4 |

|
437 | 34.4 | 438.1502 | 440.1551 | 420–281 | F |
| M5 |
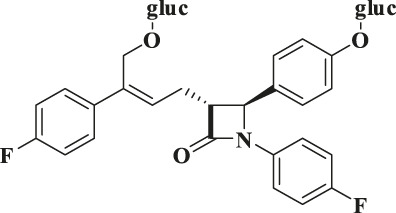
|
773 | 22.9–23.1 | 772.2068 | 774.2128 | 596–283 | P, U |
| M6 |

|
535 | 27.4 | 534.1046 | 536.1125 | 516–436–299–281 | F |
| M7 |

|
517 | 31.1 | 518.1072 | 520.1150 | 518–500–420–309–283 | F |
| M8 |

|
625 | 32.1–32.4 | 624.1692 | 626.1744 | 596–487–311–283–265 | U |
F, feces; MS, mass spectrometry; MW, molecular weight; P, plasma; U, urine.
