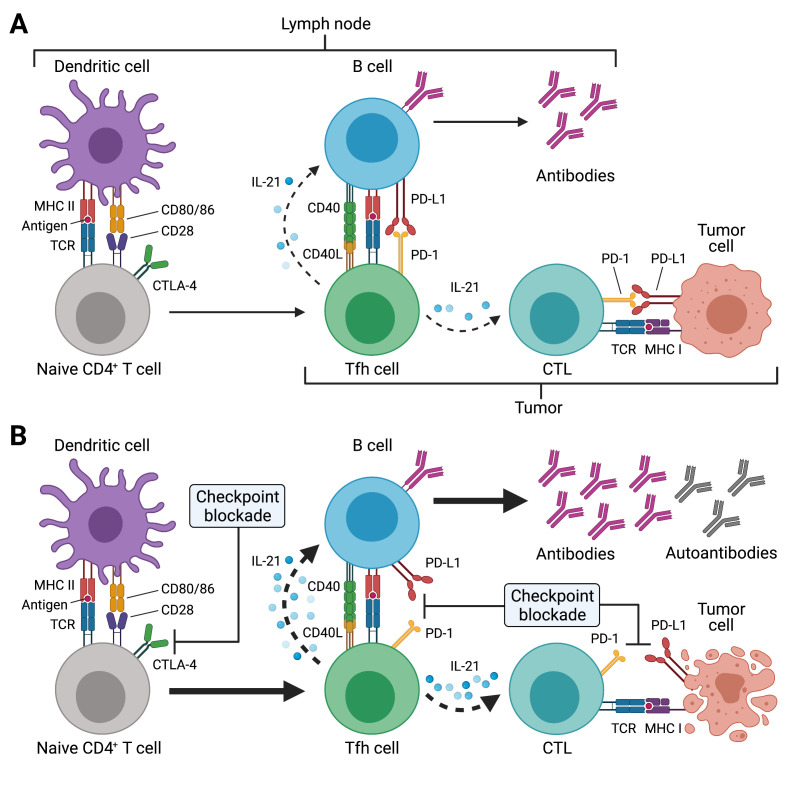
Figure 1.
Potential effects of immune checkpoint inhibitor (ICI) treatment on T follicular helper (Tfh) cell responses. (A) Tfh cell differentiation is initiated in the T cell zone of secondary lymphoid organs (SLOs) such as lymph nodes (LNs) by priming of naïve CD4+ T cells through dendritic cells (DCs). This involves presentation of antigenic peptides (eg, derived from drained tumor tissues) on major histocompatibility complex class II (MHC II) and co-stimulation through CD28, which is expressed on T cells. CTLA-4 inhibits CD28-induced proliferation and acts as a break. SLO-resident Tfh cells and tumor-resident Tfh-like cells (as indicated by the different brackets) provide critical help to B cells for antibody responses through T-cell receptor (TCR) recognition of cognate (tumor) antigens presented on MHC II. Tfh and Tfh-like cells deliver co-stimulatory (eg, CD40L) and receive co-inhibitory (eg, PD-1) signals to/from B cells and together with cytokines such as interleukin-21 (IL-21) instruct antibody diversification and affinity maturation. In the context of tumors, IL-21 may also promote the antitumor activity of cytotoxic T cells (CTLs). (B) ICI treatment acts at different stages of the Tfh cell response. Anti-CTLA-4 treatment boosts naïve CD4+ (and CD8+) T cell priming, thus resulting in highly activated and proliferating CD4+ T cells such as Tfh cells (as well as CTLs). Tfh and Tfh-like cells express high levels of PD-1 and blockade of this pathway during ICI treatment may unleash the antibody response, which may also result in the production of autoreactive antibodies. Besides its direct effect on CTLs, PD-1/PD-L1 blockade may also boost IL-21 provision to CTLs by Tfh and Tfh-like cells in LNs and tumor tissues, with the resulting exaggerated CTL response, potentially also driving autoimmune manifestations of immune-related adverse events (irAEs). Created with BioRender.com.