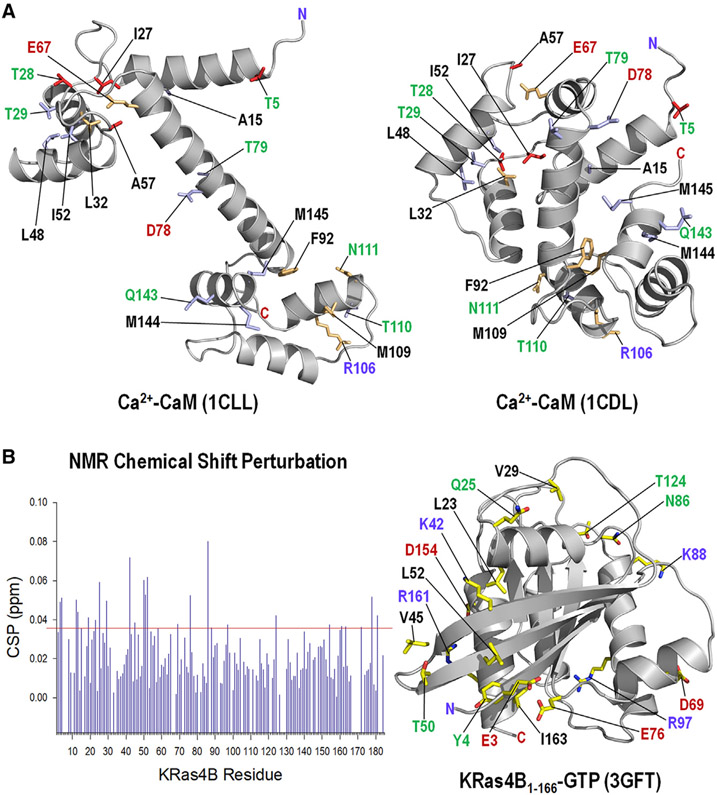
Figure 1. NMR CSPs and Mapping on the Structure.
(A) NMR CSPs of residues by truncated KRas4B1-166 (red sticks) and by full-length KRas4B1-188 (light blue sticks) mapped onto the crystal structures of CaM with a stretched central linker (PDB: 1CLL) (left panel) and a collapsed linker (PDB: 1CDL) (right panel). The light green sticks denote the perturbed residues by both truncated and full-length KRas4B.
(B) NMR CSPs of residues in full-length KRas4B1-188 in the GTP-γ-S bound state by CaM (left panel), and mapping of the perturbed residues on the crystal structure (PDB: 3GFT) of the catalytic domain of KRas4B (right panel). In the structure, hydrophobic, hydrophilic, positively charged, and negatively charged residues are marked by black, green, blue, and red letters, respectively.