Abstract
Gastrointestinal stromal tumors (GISTs) are the most important and common mesenchymal tumors of the gastrointestinal tract, especially in the stomach. GISTs are usually driven by activating mutations in either KIT or PDGFRA genes. It is known that activating gene mutations predicts, to a certain extent, not only the morphology of the tumor cells but also a response to treatment with tyrosine kinase inhibitors. Here, we present a case of an epithelioid variant of GIST harboring PDGFRA and MLH1 gene alterations in the stomach of a 55-year-old Japanese woman. The tumor of 98 mm with multiple cysts showed exophytic growth from the gastric fundus. Histopathologically, it consisted of scattered medium-sized epithelioid tumor cells in a loose myxoid background. Based on c-kit and DOG-1 immunoreactivity and a PDGFRA mutation (p.Trp559_Arg560del), the tumor was diagnosed as an epithelioid variant GIST. Interestingly, it had a gene alteration (p.Met524Ile) in the MLH1 gene of unknown pathogenicity. It was assigned to Group 3a (low risk for malignant behavior). After surgery, the patient has been on imatinib therapy and disease-free for 10 months.
Keywords: epithelioid variant, GIST, MLH1, PDGFRA, stomach
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most important and common mesenchymal tumors of the gastrointestinal tract with an estimated annual incidence of 14–20 cases per million. GISTs generally present as sporadic disease in elderly people with no sex differences.1,2 They are commonly driven by activating mutations in either KIT or PDGFRA receptor tyrosine kinase genes in a mutually-exclusive manner. Hereditary KIT and PDGFRA mutations cause familial GIST syndromes.3,4 It is well known that GIST may occur in patients with other hereditary tumor syndromes, such as the neurofibromatosis, Carney triad, and Carney-Stratakis syndrome.4–6 In the latter two conditions, GISTs presenting an epithelioid morphology and such GISTs can occur in young age. Genetically, this type of GISTs may present a loss-of-function in the succinate dehydrogenase complex without KIT and PDGFRA gene mutations.6
In KIT-mutant GISTs, exon 11 mutations are most common. A deletion of KIT gene causes a more aggressive phenotype than its base substitution. KIT exon 11 mutants were reported to respond well to imatinib, a tyrosine kinase inhibitor.3 PDGFRA mutations are found in only 5% of GISTs and are thus much less common than KIT mutations. The most common PDGFRA gene mutation of GISTs is an Asp842Val substitution in exon 18. These PDGFRA-mutant GISTs commonly occur in the stomach, and have a predominantly epithelioid or mixed epithelioid/spindle cell morphology.3
Here, we report a case of an epithelioid variant of GIST harboring PDGFRA mutation and MLH1 gene alteration of unknown pathogenicity, which occurred in the stomach of a 55-year-old Japanese woman.
CLINICAL SUMMARY
A 55-year-old Japanese woman visited our hospital, complaining of upper abdominal distension. Her physical examination revealed a large palpable mass on the left upper abdomen though the laboratory values were not abnormal. The upper gastrointestinal endoscopy revealed a large submucosal tumor on the gastric fundus (Figure 1a). The colonoscopy revealed no polyps in the colorectum. The computed tomography showed a 95 mm, multicystic tumor with contrast enhancement on the upper left abdomen (Figure 1b and c). The lesion was clinically and radiographically diagnosed as GIST in the stomach. A wedge resection of the greater curvature of the stomach was performed. The postoperative course of the patient was uneventful under imatinib treatment, without recurrence for 10 months.
Figure 1.
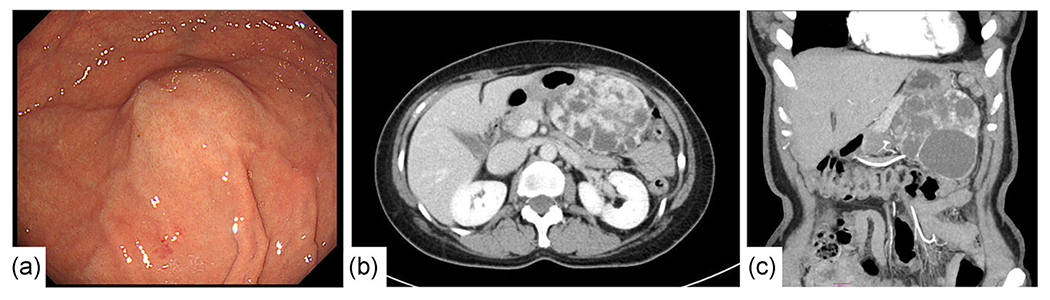
Upper gastrointestinal endoscopy (a) and contrast-enhanced computed tomography (b,c). (a) A smooth submucosal tumor without ulceration on the gastric fundus. (b,c) (b) axial; (c) coronal. A round, well-margined multicystic tumor measuring 98 mm in the maximal diameter at the left upper abdomen showing contrast .enhancement.
PATHOLOGICAL FINDINGS
The size of the resected tumor was 98 × 85 × 60 mm. The cut surface was yellowish with a spotty hemorrhagic area and multiple cysts (60 mm in the diameter of the maximum cystic lesion). The tumor was encapsulated by a thin fibrous layer and the surgical margins were free of tumor (Figure 2). Histopathologically, the tumor consisted of scattered middle-sized epithelioid tumor cells within a loose myxoid background. The tumor cells contained abundant eosinophilic cytoplasm and moderately enlarged vesicular nuclei with a few small nucleoli. Binucleated tumor cells were also present (Figure 3a). The mitotic rate was low (1 per 5 mm2). Although liquefactive necrosis with lymphoid infiltration were present (Figure 3b), coagulating necrosis as well as lining epithelial cells on the cyst wall were not found.
Figure 2.
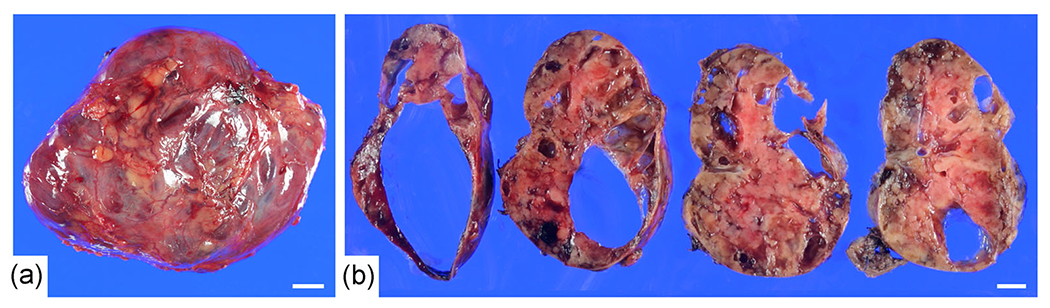
Gross findings of the surgically-resected specimen. (a) The size of the resected tumor was 98 × 85 × 60 mm. The tumor was encapsulated by thin fibrous tissue. (b) The cut surface was yellowish with spotty hemorrhagic areas and multiple cysts (up to 60 mm in the maximum diameter). Bar, 1 cm.
Figure 3.
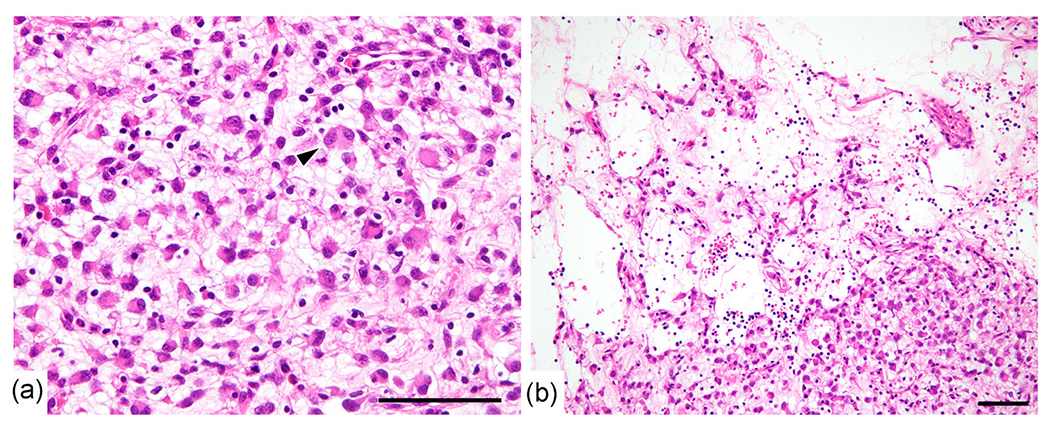
Histological findings. (a) The tumor consisted of scattered middle epithelioid tumor cells with abundant eosinophilic cytoplasm and moderately enlarged vesicular nuclei within a loose myxoid background. A binucleated tumor cell (arrow head) was present. (b) Liquefactive necrosis with lymphoid infiltration was observed. Bar, 100 μm.
Representative immunohistochemistry images are presented in Figure 4, revealing scattered c-KIT-positive tumor cells with focal DOG-1 positivity but no expression of CD34. Desmin was strongly positive. Weak α-smooth muscle actin (SMA) expression was observed in a minor population. There was no immunoreactivity for pan-cytokeratin, S-100 protein, HMB45, and no markers of neuroendocrine differentiation were observed. The Ki-67 labeling index was 4.5% (45 positive tumor cells per 1000 tumor cells). MLH1 and PMS2 expression was preserved.
Figure 4.
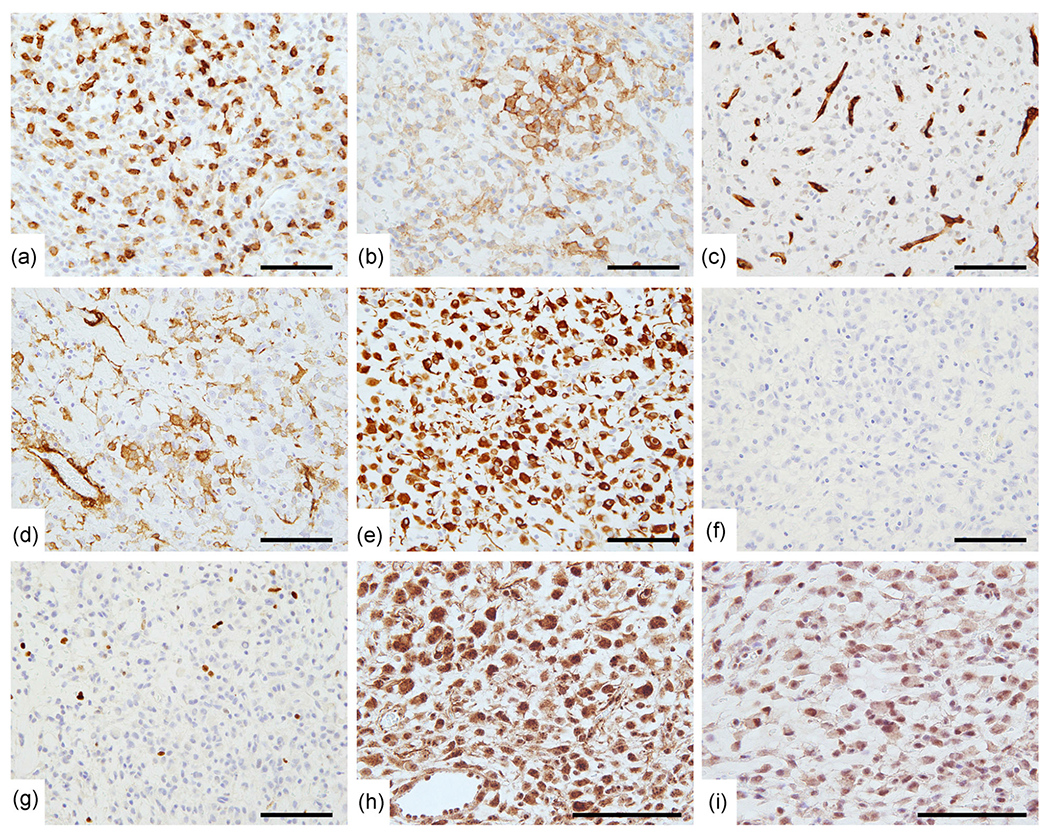
Immunohistochemical findings. Representative immunohistochemistry for (a) c-KIT, (b) DOG-1, (c) CD34, (d) α-smooth muscle actin, (e) Desmin and (f) HMB45. The Ki-67 labeling index was 4.5% (g). MLH1 (h) and PMS2 (i) expression was preserved. Note that immunohistochemical staining for MLH1 (1:200 dilution, clone G168-728, BD Biosciences, Franklin Lakes, NJ) and PMS2 (1:50 dilution, clone A16-4, BD Biosciences) was performed using the Ventana BenchMark XT automated immunostainer and OptiView reagent (Roche Diagnostics, Basel, Switzerland). Bar, 100 μm.
Formalin-fixed paraffin-embedded specimens were subjected to next-generation sequencing (NGS) analysis using the Ion Torrent platform. The analysis revealed the presence of PDGFRA deletion (c.1675_1680del p.Trp559_Arg560del), whose allele frequency was 25.3%, and MLH1 gene alteration (c.1572 G > C p.Met524Ile), whose allele frequency was 50.5% (Figure 5a and c). These results were confirmed by Sanger sequencing (Figure 5b and d). No mutations of KIT exons 8, 9, 11, 13, 14, 17, and 18 were identified by Sanger sequencing. Also, exons 14 and 18 of PDGFRA were found to have a wild-type sequence (data not shown).
Figure 5.
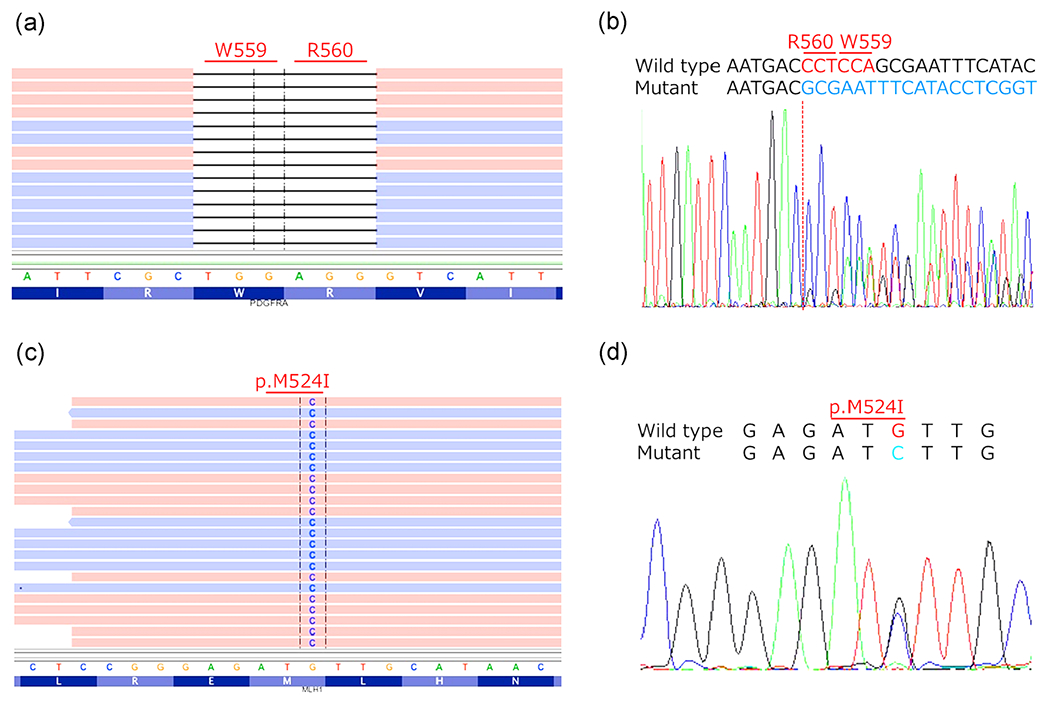
Mutational analysis by next-generation (a,c) and Sanger sequencing (b,d). PDGFRA c.1675_1680del p.Trp559_Arg560del (A and B) and MLH1 c.1572 G > C p.Met524Ile gene alterations (c and d) were identified. Note that red and blue colors in NGS sequence indicate forward and revers reads, respectively.
Based on c-KIT and DOG-1 immunoreactivity along with PDGFRA mutations, a diagnosis of epithelioid variant GIST was made. This case was assigned to Group 3a (low risk for malignant behavior).7
DISCUSSION
Differential diagnosis of GISTs includes many neoplasms, mainly mesenchymal tumors. In diagnosing GIST composed of epithelioid tumor cells, it is necessary to carefully rule out adenocarcinomas, epithelioid leiomyomas/leiomyosarcomas, epithelioid type PEComas, glomus tumors, carcinoids (neuroendocrine tumors) and melanomas.8 In the present case, the possibility of adenocarcinoma, neuroendocrine tumor, PEComa, and melanoma was excluded based on the lack expression of pan-cytokeratin, S-100 protein, neuroendocrine markers, and HMB45. A glomus tumor was also ruled out by strong desmin expression. Although desmin can rarely be expressed (< 9%) in GISTs including PDGFRA-mutated ones,9,10 tumors of myoid differentiation should be carefully ruled out in this case due to the strong desmin and weak α-SMA expression. Mutation analyses using next-generation and Sanger sequencing identified PDGFRA exon 12 deletion. Finally, a diagnosis of epithelioid variant of GIST was made according to the sparse KIT and focal DOG-1 expression, together with PDGFRA mutation.
Mutational activation of the PDGFRA gene has been reported in 5% of GISTs. PDGFRA mutations occur almost exclusively in GIST of the stomach that have a tendency to show epithelioid or mixed epithelioid/spindle cell morphology.3 In GISTs, single nucleotide substitutions are most common in exons 12, 14, and 18 of the PDGFRA gene. Over a half of all PDGFRA single nucleotide alterations involve amino acid changes in exon 18, D842V. In-frame deletions, as in the present case, are the second most common.3 These deletion mutations have been identified in exons 12 and 18 of PDGFRA. The Catalogue of Somatic Mutations in Cancer (COSMIC, https://cancer.sanger.ac.uk/cosmic) database includes a case of GIST carrying an identical PDGFRA exon 12 deletion (p.Trp559_Arg560del).11 Interestingly, in spite of the identical PDGFRA exon 12 deletion, there were several histopathological differences. The major one was that, whereas GIST presented diffuse DOG-1 expression without desmin expression in the previous report, it presented focal DOG-1 expression with diffuse strong desmin expression in our case. That patient suffered recurrent tumors in the peritoneal cavity and liver after surgery despite having no additional mutations in the KIT and PDGFRA genes and despite receiving neoadjuvant imatinib treatment. Interestingly, these metastases showed a striking response to systemic sunitinib treatment, resulting in complete resolution. In our case, no metastases or recurrent tumors have been identified up to the time of submission of this case report. However, should there be later recurrence, sunitinib could be considered as a systemic therapy.
MLH1 is the human homolog of the Escherichia coli gene, mutL, which plays key roles in repairing DNA mismatches. Defects of DNA mismatch repair genes including MLH1 are associated with the microsatellite instability (MSI) observed in Hereditary Non-Polyposis Colorectal Cancer (HNPCC). In GISTs, infrequent methylation of the MLH1 gene has been reported,12 but to the best of our knowledge, no MLH1 mutations have been previously reported. In the present case, MLH1 c.1572 G > C transversion was found in exon 14, one of the exons serving as constitutive PMS2 dimerization domains, at an allele frequency of 50.5%.13 This high frequency of altered allele might indicate germline not but somatic alterations. This type of MLH1 gene alteration is not documented in the COSMIC or InSiGHT variant databases (https://www.insight-group.org/variants/databases/). On one hand, this gene alteration was documented as uncertain significance in ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/). On the other, the possible impact of this gene alteration was predicted to be benign by the Polymorphism Phenotyping v2 program (PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/). Although the present case showed retained MLH1 and PMS2 expression and had a microsatellite-stable phenotype (data not shown), the pathogenesis of MLH1 c.1572 G > C gene alteration is yet to be clarified.
GISTs typically form a regular, soft, solid mass, but they may also rarely present as cyst-predominant lesions. GISTs with large cystic changes tend to grow exophytically from the gastrointestinal tract wall, and in some cases can be over 10 cm in diameter.14–16 It appears that cystic changes are more frequently observed in high-grade GISTs, possibly due to the poor blood supply to the aggressively growing tumor. It was also reported that liquefactive necrosis, scattered empty spaces with lymphoid infiltration and loose hyaline material, can be observed in nearly half of GIST tumors, but without prognostic significance.5 In the present case, proliferating cells were infrequent as reflected in the rarity of mitotic figures and lower Ki-67 labeling index. Furthermore, coagulation necrosis was not seen. Therefore, the cystic lesions of the present case were considered to be formed by liquefactive necrosis.
In summary, here we report a case of gastric GIST of epithelioid morphology with PDGFRA exon 12 deletion and MLH1 gene alteration. The patient has been free of disease under imatinib treatment for 10 months after surgery. In case of recurrence, sunitinib treatment may be considered.
ACKNOWLEDGMENT
We had the support on the manuscript editing from Ms. Yukiko Kuru (Aichi Medical University).
Abbreviations:
- GIST
gastrointestinal stromal tumor
- MSI
microsatellite instability
- HNPCC
Hereditary Non-Polyposis Colorectal Cancer
Footnotes
DISCLOSURE STATEMENT
None declared.
REFERENCES
- 1.Goettsch WG, Bos SD, Breekveldt-Postma N, Casparie M, Herings RMC, Hogendoorn PCW. Incidence of gastrointestinal stromal tumours is underestimated: results of a nation-wide study. Eur J Cancer 2005; 41: 2868–72. [DOI] [PubMed] [Google Scholar]
- 2.Tryggvason G, Gíslason HG, Magnússon MK, Jónasson JG. Gastrointestinal stromal tumors in Iceland, 1990-2003: the icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer 2005; 117: 289–93. [DOI] [PubMed] [Google Scholar]
- 3.Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology 2008; 53: 245–66. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen M, Lasota J. Histopathology of gastrointestinal stromal tumor. J Surg Oncol 2011; 104: 865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 2005; 29: 52–68. [DOI] [PubMed] [Google Scholar]
- 6.Miettinen M, Wang ZF, Sarlomo-Rikala M, Osuch C, Rutkowski P, Lasota J. Succinate dehydrogenase-deficient GISTs: a clinicopathologic, immunohistochemical, and molecular genetic study of 66 gastric GISTs with predilection to young age. Am J Surg Pathol 2011; 35: 1712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006; 23: 70–83. [DOI] [PubMed] [Google Scholar]
- 8.Hirota S Differential diagnosis of gastrointestinal stromal tumor by histopathology and immunohistochemistry. Transl Gastroenterol Hepatol 2018; 3: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 2001; 438:1–12. [DOI] [PubMed] [Google Scholar]
- 10.Lasota J, Dansonka-Mieszkowska A, Sobin LH, Miettinen M. A great majority of GISTs with PDGFRA mutations represent gastric tumors of low or no malignant potential. Lab Invest 2004; 84: 874–83. [DOI] [PubMed] [Google Scholar]
- 11.Brohl AS, Demicco EG, Mourtzikos K, Maki RG. Response to sunitinib of a gastrointestinal stromal tumor with a rare exon 12 PDGFRA mutation. Clin Sarcoma Res 2015; 5: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igarashi S, Suzuki H, Niinuma T et al. A novel correlation between LINE-1 hypomethylation and the malignancy of gastrointestinal stromal tumors. Clin Cancer Res 2010; 16: 5114–23. [DOI] [PubMed] [Google Scholar]
- 13.Rosty C, Clendenning M, Walsh MD et al. Germline mutations in PMS2 and MLH1 in individuals with solitary loss of PMS2 expression in colorectal carcinomas from the Colon Cancer Family Registry Cohort. BMJ Open 2016; 6: e010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naitoh I, Okayama Y, Hirai M et al. Exophytic pedunculated gastrointestinal stromal tumor with remarkable cystic change. J Gastroenterol 2003; 38: 1181–84. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Liu L, Liu Z, Tian Y, Lin Z. Giant gastrointestinal stromal tumor with predominantly cystic changes: a case report and literature review. World J Surg Oncol 2017; 15: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu CC, Liu Y, Zhao G. Exophytic gastrointestinal stromal tumor with cystic changes: A case report. Oncol Lett 2014; 7: 1427–29. [DOI] [PMC free article] [PubMed] [Google Scholar]


