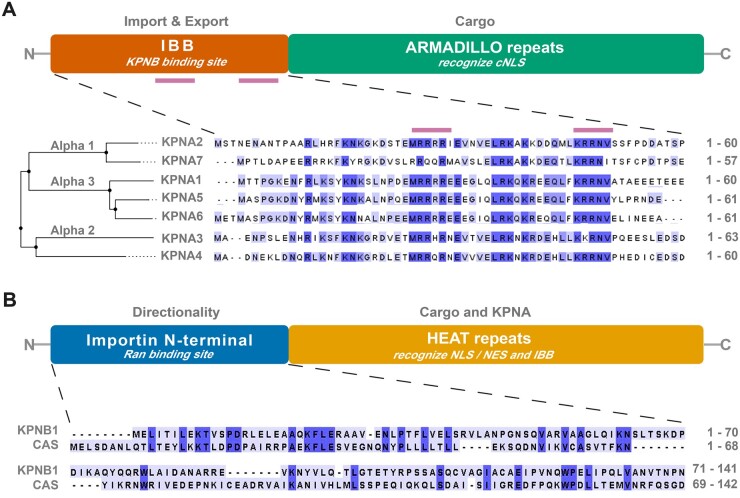
Figure 1.
Functional architecture of karyopherins. (A) KPNA family proteins consist of two main functional domains: an importin beta (KPNB) binding (IBB) domain for nuclear import and export and armadillo repeats for the recognition of canonical NLS (cNLS) of cargo proteins. The IBB consists of cNLS binding boxes (bars in purple), major (‘KRR’) and minor (‘RRRR’ or ‘RRQR’ or ‘RRHR’) binding sites, which in the absence of KPNB occupy the canonical NLS-binding surface of armadillo repeats. This prevents import of ‘unloaded’ karyopherin complexes in the nucleus. KPNA family proteins are subdivided into three subfamilies, α1, α2 and α3, based on differences in amino acid sequence in their IBB domain and cNLS binding sites. See Table 1 for details on all KPNA family members. (B) KPNB family proteins comprise an N-terminal (20–120 amino acids) importin domain responsible for binding with Ran and for directed protein translocation across the nuclear envelope, and HEAT repeats that are distinguishable by their flexibility and recognition/binding of cargo-proteins via the NLS or the nuclear export signal and binding to KPNA. Shown are two functionally important representatives of the KPNB family, KPNB1 and CAS (see Table 2 for details on all KPNB family members). Protein sequences were obtained from UniProt (see Tables 1 and 2 for ID numbers); Clustal Omega was used for sequence alignment. CAS = cellular apoptosis susceptibility protein; NES = nuclear export signal.