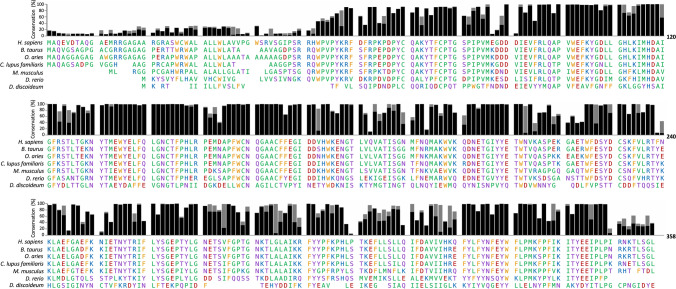
Fig. 3.
Mature CLN5 is highly conserved throughout species. Black bars represent the percent conservation of each human CLN5 residue across the 397 CLN5 protein sequences available on UniProt. Stacked grey bars represent the percent conservation of amino acids with similar chemical properties. Underneath are the aligned amino acid sequences for CLN5 from the model organisms used to study CLN5. Amino acids are coloured according to their chemical properties: non-polar residues (G, A, V, C, P, L, I, M) are in green; polar, uncharged residues (S, T, Y, N, Q) are in purple; basic, positively charged residues (K, R, H) are in blue; acidic, negatively charged residues (D, E) are in red; and aromatic residues (W, F) are in yellow. Created using Microsoft Excel and Powerpoint