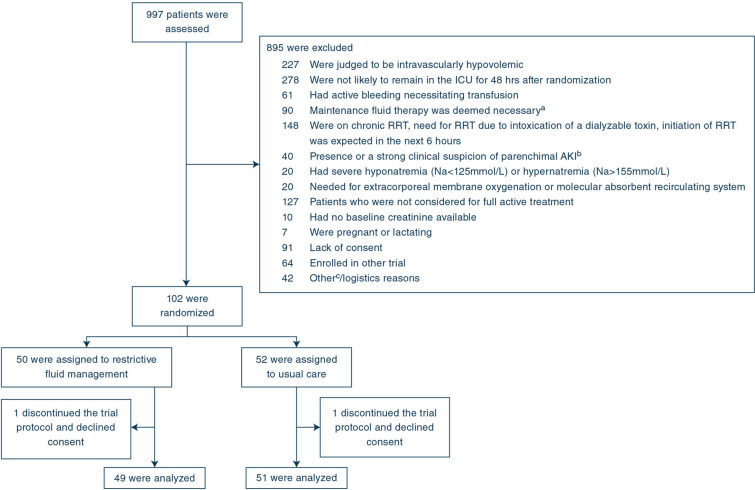
Fig. 1.
Flowchart of trial patients. ICU intensive care unit, RRT renal replacement therapy. a Diabetic ketoacidosis, non-ketotic coma, severe burns or other clinical reason determined by the medical staff. b Glomerulonephritis, vasculitis, acute interstitial nephritis, or post-renal obstruction. c Including two exclusion criteria that were removed in a protocol amendment in April 2018 (1) Metformin-induced lactic acidosis or acute liver failure (n = 8) (2) AKI stage 2 or greater is known to have been present for > 48 h (n = 14). These criteria were amended to clarify the exclusion criteria