Figure 2: A graphic summary of targets in cancer cells altering IP3R-mediated ER-mitochondrial Ca2+ fluxes.
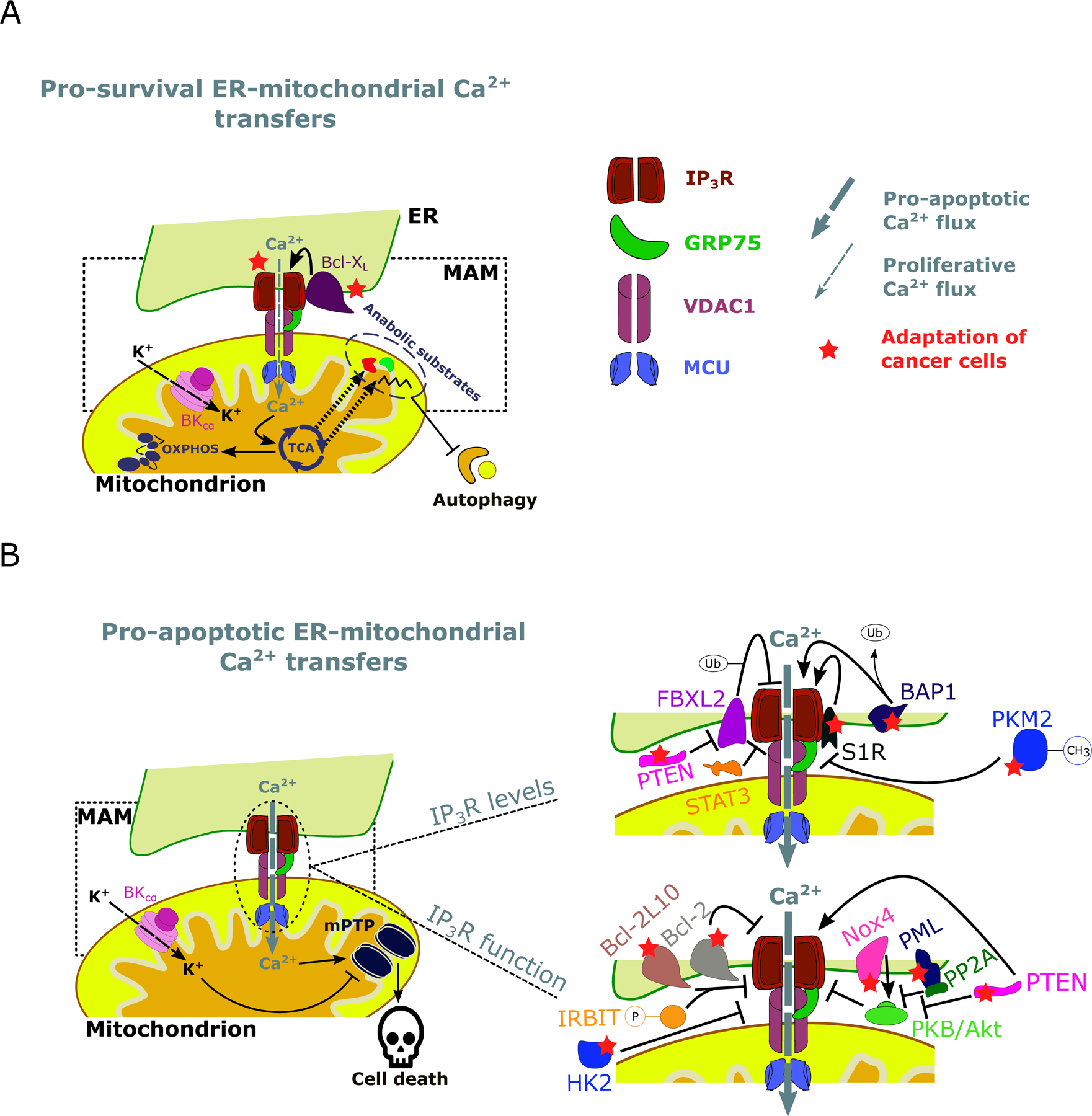
A: Cancer cells are addicted to pro-survival ER-mitochondrial Ca2+ transfers, which promote the mitochondrial metabolism through Ca2+-dependent stimulation of TCA and OXPHOS and suppress autophagic flux. This not only serves for ATP production but also for anabolic pathways through the production of intermediate substrates to synthesize nucleotides. IP3R function can be enhanced by anti-apoptotic/pro-survival proteins such as Bcl-XL. B: Cancer cells also avoid pro-apoptotic ER-mitochondrial Ca2+ transfers. Excessive Ca2+ levels in the mitochondrial matrix lead to cell death via opening of the mPTP. Cancer cells can circumvent this by i. decreasing IP3R levels or ii. modulate IP3R function. PTEN competes with FBXL2, which can mark IP3R for degradation by ubiquitination. Similarly, the oncogenic transcription factor STAT3 facilitates IP3R degradation at the MAMs. Conversely, BAP1 interacts with and deubiquitinates IP3R. Additionally, S1R binds and stabilizes IP3R. Methylated PKM2 was observed to interact with IP3R in the MAMs and to promote IP3R downregulation. Note that the methylations of PKM2 are more complex than the single methyl group depicted here. PTEN negatively regulates PKB/Akt-dependent phosphorylation of IP3Rs, this way promoting pro-apoptotic Ca2+ signaling in the MAMs. Additionally, Nox4 promotes PKB/Akt-mediated inhibitory IP3R phosphorylation. Also, PML recruits PP2A to the MAMs, where it counteracts PKB/Akt activity. Moreover, several anti-apoptotic Bcl-2 proteins can inhibit IP3R, either directly or in cooperation with phosphorylated IRBIT (as is the case for Bcl-2L10). Finally, HK2 negatively modulates pro-apoptotic IP3R-driven Ca2+ fluxes. ER: Endoplasmic reticulum, MAM: Mitochondria-associated ER membrane, TCA: Tricarboxylic acid cycle, OXPHOS: oxidative phosphorylation, Ca2+: Calcium ion, K+: Potassium ion, IP3R: Inositol 1,4,5-trisphosphate receptor type 1, GRP75: Glucose-regulated protein 75, VDAC1: Voltage dependent anion channel 1, MCU: Mitochondrial Ca2+ uniporter, BKCα: Calcium-activated potassium channel, Bcl-XL: B-cell lymphoma extra-large, mPTP: Mitochondrial permeability transition pore, FBXL2: F-box/LRR-repeat protein 2, Bap1: Ubiquitin carboxyl-terminal hydrolase BAP1, PTEN: Phosphatase and tensin homolog, STAT3: Signal transducer and activator of transcription 3, S1R: Sigma-1 receptor, Bcl-2: B-cell lymphoma 2, Bcl-2L10: Bcl-2 like protein 10, Nox4: NADPH oxidase 4, PML: Promyelocytic leukemia protein, PP2A: Protein phosphatase 2A, IRBIT: IP3R binding protein released with IP3, PKB/Akt: Protein kinase B, HK2: Hexokinase 2, Ub: Ubiquitinylations, P: Phosphorylations, CH3: Methylations.
