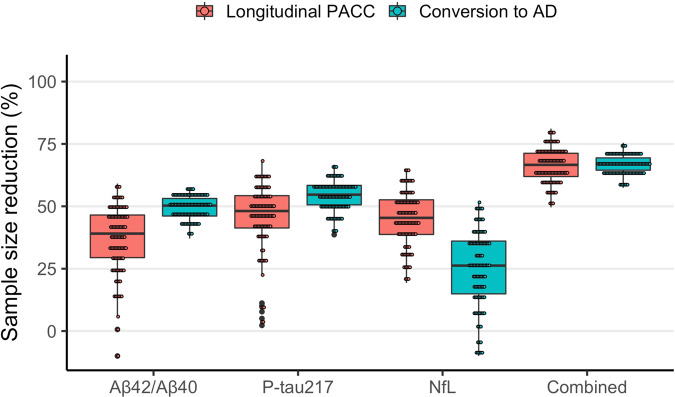
Fig. 3. Power enrichment analysis of plasma biomarkers in a theoretical clinical trial.
This figure shows the reduction in sample size resulting from using plasma biomarkers for inclusion enrichment in theoretical clinical trials aimed at slowing decline in PACC or reducing risk of AD dementia in a CU population. Sample sizes were estimated for a trial enriched using pre-defined cutoffs for each biomarker as inclusion threshold. Confidence intervals were derived by calculating the 0.025% and 95.75% percentile values from 1000 bootstrapped trials over n = 435 individuals. Source data is available in Supplementary Table 8.