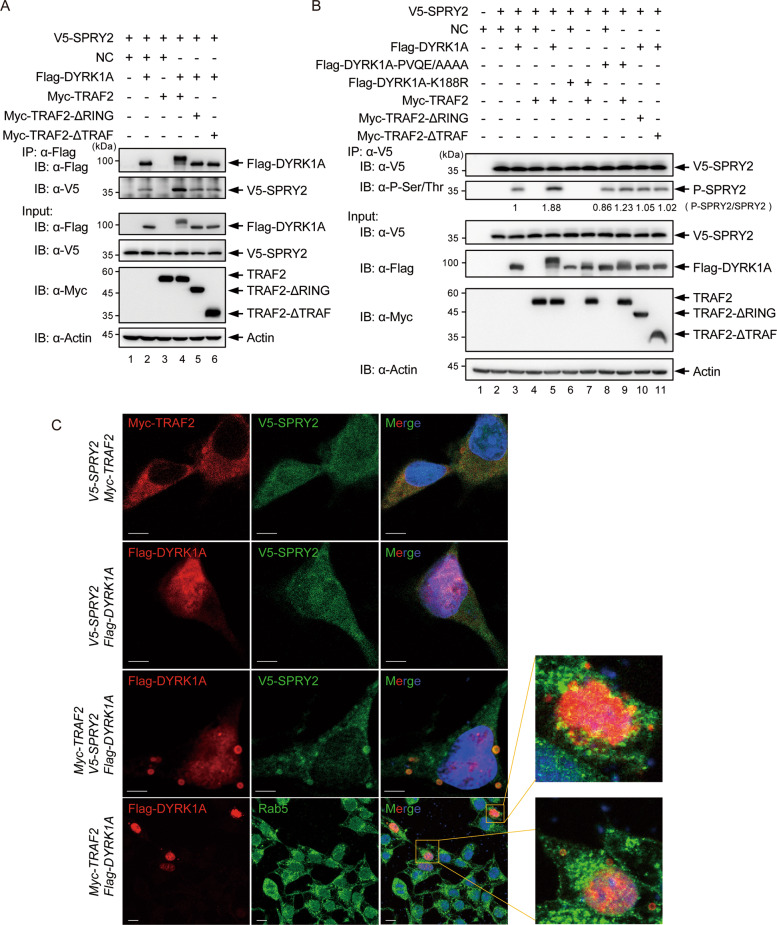
Fig. 5. TRAF2 promotes DYRK1A-mediated phosphorylation of SPRY2.
A Interaction analysis between DYRK1A and SPRY2 in the presence of WT TRAF2 or its truncates. V5-SPRY2 was co-transfected with Flag-DYRK1A, Myc-TRAF2, or its different truncations into HEK293T cells and Flag-affinity purified. Immunoprecipitates were probed with α-Flag and α-V5 to analyze the enrichment of SPRY2 in DYRK1A pulldowns after overexpression of TRAF2 and truncates. B Analysis of phosphorylation status of SPRY2 in the presence of DYRK1A and TRAF2. V5-SPRY2 was co-transfected with Flag-DYRK1A (WT, PVQE/AAAA or K188R) and/or Myc-TRAF2 (WT, ΔRING or ΔTRAF) in HEK293T cells, followed by V5-affinity purification. Immunoprecipitates were probed with α-phospho-pan-Ser/Thr antibody. Full-length TRAF2, but not ΔRING or ΔTRAF deletions, was able to promote phosphorylation of SPRY2 in the presence of wild-type DYRK1A. Also, Flag-DYRK1A-PVQE/AAAA mutant did not promote the phosphorylation of SPRY2. C Cellular localization of V5-SPRY2, Myc-TRAF2, Flag-DYRK1A, and Rab5. HEK293T cells were co-transfected with V5-SPRY2, Flag-DYRK1A, and/or Myc-TRAF2, and immunostained with respective antibodies. Images were acquired using ZEISS 710 confocal laser scanning microscope. The first three panels, scale bar = 25 μm. The last panel, scale bar = 1 μm.