Abstract
The objective of the present investigation was to identify and characterize the pigment produced by yeast strain Rhodotorula mucilaginosa (MTCC-1403) using food industry residues. Onion peel powder and Mung bean husks were explored as substrate for submerged fermentation at previously optimized conditions in 3-L bioreactor. The pigment extraction was followed by quantification and characterization in terms of UV–visible spectroscopy, Fourier transform infrared spectroscopy, high performance liquid chromatography and fluorescence spectroscopy. Anti-carcinogenic activity of extracted pigment was measured against MCF-7 breast cancer cells. Furthermore, the pigment was used for the development of confectionary products (hard boiled candy and jelly) at different concentrations to evaluate its influence on bioactive properties and functionality. UV–visible spectroscopic reports revealed that torularhodin, β-carotene, and torulene were major carotenoids present. In case of anti-carcinogenic activity, cell inhibition of 21.21% was observed with 40 μg of the extracted pigment after 72 h of incubation against MCF-7 cells. Significant influence of extracted pigment on confectionary products was observed for antioxidant activity, carotenoid content, color profile and sensory evaluation.
Keywords: Rhodotorula mucilaginosa, Agro-industrial waste, Carotenoids, Antioxidant activity
Introduction
Color has been referred to as prime parameter to dictate the quality of any food product. The necessity for food colorants arises from the fact that appearance is the fundamental attribute to evaluate the quality of any given product. The objective of food colorants is either to substitute the color eluded during processing or augment the one already present. There are plenty of artificial pigments which are being contrived and exploited in the food industries (Tuli et al. 2015) that may cause severe harmful effects on human health. Simulated colors are criticized for their application in food products due to their neurotoxic, carcinogenic and genotoxic properties (Hayashi et al. 2000). Positive adaptability and consumers priority towards the use of natural products is encouraging the industries to use biological pigments. Although natural colors are widely distributed in our surroundings, cost of the extraction and purification is the major constraint. Joshi et al. (2003) reported that biological colors are not cost effective due to their expensive market value, lower stability and lesser potency and intensity.
Pigments of biological origin are rich in bio-functional compounds having antioxidant capacity owing to their potential role against free radicals generated through oxidative stress which may otherwise trigger serious harm to the cells leading to inception of chronic complications (Rabeta et al. 2013; Guaadaoui et al. 2014). Thus, antioxidant rich compounds are known to have pivotal role in prevention of several modern lifestyle disorders such as neurological concerns, cancers and cardiac diseases.
Carotenoids are a class of phytonutrients comprising of several bioactive compounds which induce beneficial effects to the human health. They act as provitamin A and their antioxidant potential reduces the risk of certain abnormalities and helps in the enhancement of the immune system (Kim et al. 1997). They address several other health-related afflictions such as prevention of CVDs, inhibition of free radicals and prophylaxis of skin degradation due to the exposure to UV light (Britton et al. 2008). Although carotenoids have several promising health benefits, the cost-effectiveness of production is still the major constraint. Microbial synthesis of pigments can be an economic approach in comparison to the plant sources if optimum conditions are met.
Yeasts have the ability to synthesize carotenoids when grown in medium containing refined carbon sources such as glucose, sucrose, glycerol, and sorbitol (Polulyakh et al. 1991; Parajó et al. 1998; ZHANG WSZX, Kai HL 2001; Frengova et al. 2004). Recent research trends have focused on the use of low-cost carbon sources such as grape juice, legume hydrolysates like mung bean flour and mustard waste isolates and corn hydrolysates (Martin et al. 1993; Cruz and Parajo 1998; Kesava et al. 1998; Buzzini and Martini 2000). Rhodotorula species and Phaffa species are the most significant classes of yeasts producing microbial pigments particularly carotenoids. Phaffa rhodozyma have been reported to produce astaxanthin, a red plant pigment rarely found in microorganisms (Chreptowicz et al. 2019). Sporobolomyces roseus has been studied for the production of tetraterpenoids and a new class of Cystobasidium genus is also under observation. Basidiomycetes species of yeast have the ability to utilize urea in contrast to Ascomycetous yeasts (Chreptowicz et al. 2019). Other yeasts include Cryptococcus species and Saccharomyces species. Sporadic reports are available exploring the potential of R mucilaginosa for carotenoid production and applications in food products have not been documented.
Thereby, present investigation was undertaken with two objectives: a) to identify and characterize the pigment extracted from R mucilaginosa produced by fermentation of agro-industrial residues under pre-optimized environmental conditions and b) to study the anti-carcinogenic activity and functionality of extracted pigment in confectionary products.
Material and methods
Microbial culture and substrate preparation
Rhodotorula mucilaginosa MTCC-1403 (yeast strain) was obtained from Institute of Microbial Technology, Chandigarh, India. The yeast was grown on Yeast Malt Extract (YME) agar plates and incubated at 25 °C for two days. Plates were stored at refrigerated conditions and later sub-culturing was carried out after interval of every fifteen days.
For economic production of pigment, substrates from agro-industrial origin were used. Onion peel (OP) powder and Mung Bean Husk (MBH) were taken as agro-industrial waste. For preparation of aqueous extracts, equal amounts of OP and MBH powders (one part) were suspended in distilled water (five parts) followed by autoclaving. The slurry was then filtered, and centrifugation was done at 10,000×g for 20 min. The clear supernatant was used as agro-industrial waste substrate for further fermentation batches (Nancib et al. 2015).
Fermentation procedure and conditions
Fermentation was done in 3L stirred tank bioreactor (Biospin 3A Fermenter Bioage Equipments, Mohali, India). Aqueous extract of OP and MBH were used as the substrate for fermentation at pre-optimized conditions (Temperature-26 °C, pH-6.2, agitation- 120 rpm and aeration- 1.0 vvm) (Sharma and Ghoshal 2020). 3% inoculum was added manually through the inoculum port provided at top of the flange of the vessel under flame. The fermentation was carried out for 120 h and the sample was taken out after every 12 h from the vessel for further analysis.
Extraction and estimation of carotenoids
Carotenoids were extracted by method used by Sharma and Ghoshal (2020). Yeast cell pellets were washed and lysed in 1 M HCl solution (1:10) in a water bath at 60 °C for 10 min followed by extraction with ethanol. Estimation of total carotenoids was done by UV–visible Spectrophotometric method as suggested by Cheng and Yang (2016). The maximum absorbance was observed at 490 nm and the amount of total carotenoids was estimated using Eq. 1.
| 1 |
Amax—the absorbance at 490 nm. D—Sample dilution ratio. V—Volume of extraction solvent (mL). E—Extinction coefficient of total carotenoids (0.16). W—Dry weight of yeast (g).
Characterization of extracted pigment
UV–visible spectrophotometer
The extracted pigment was analyzed to confirm the presence of carotenoids by UV–visible spectrophotometer (UV-1900model, Shimadzu, Kyoto, Japan) at a wavelength ranging between 200 and 800 nm to determine the peaks and maximum absorbance (λmax) value of the band. The target region was the fingerprint region of the carotenoids i.e. 400–500 nm. Measurement was made against solvent blank base line.
High-performance liquid chromatography (HPLC)
High-Performance Liquid Chromatography (Shimadzu, Japan) was used to characterize the extracted pigment in terms of β-carotene. The mobile phase used in the analysis was methanol: acetonitrile: ethyl acetate (76:12:12, v/v). The pigment was extracted in ethyl acetate for equilibrium with the base line. The investigation was carried out isocratically at ambient temperature 25 °C using Merk C-18 column (150 nm × 4.6 nm, particle size 5 microns). The mobile phase solvents were initially degassed using bath sonicator (PCI, Mumbai, India) for 15 min. The flow rate of the mobile phase was kept constant at 2 mL/minute. The PDA detector was set at a wavelength corresponding to the β-carotene (450 nm). 20µL sample was injected into the instrument. Both the standard β-carotene and extracted pigment were run under similar conditions. The confirmation about the presence of β-carotene was observed by retention times and spiking of extracted pigment with standard β-carotene (Jain et al. 2016).
Fourier transform infrared (FTIR) spectroscopy
FTIR Spectroscopy was employed to further characterize the extracted pigment in terms of functional groups. FTIR spectrum of standard β-carotene and extracted pigments were recorded at 28 ± 2 °C using Tensor 27 Spectrophotometer (Bruker, Germany) in the range of 600–4000 cm−1 by accumulating 16 scans at 4 cm−1 resolution. For measuring the peak intensity of the sample, ATR (Attenuated Total Reflectance) from Bruker, Germany was used. A drop of sample was placed on ATR for measurement of peak intensity and the entire procedure for obtaining the FTIR spectrum took one minute per sample. After the spectrum was obtained, the ATR was cleaned with isopropyl alcohol before placing the next sample for analysis.
Fluorescence spectroscopy
Auto-fluorescence of the extracted pigment was measured using spectro-fluorimeter (Shimadzu, Japan). For the emission spectrum of the extracted pigment, the excitation wavelengths of 450 nm, 485 nm, and 490 nm were analyzed. The bandwidth of 0.6 nm and the step size of 0.2 nm were set for the emission.
Anti-carcinogenic activity of extracted pigment
The anti-carcinogenic activity of extracted pigment was analyzed using a method adopted by Andrade et al. (2016) with MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide] reduction method. The MCF-7 (breast cancer) cells were maintained in Roswell Park Memorial Institute (RPMI) culture medium. The medium was supplemented with 10% fetal bovine serum and 1% antibiotic solution (Penicillin and Streptomycin). The cells were maintained in an incubator at 37 °C in an atmosphere enriched with 5% CO2. The MCF-7 (105 cells/mL) cells were plated and incubated for 24 h. Pigment extracted from R mucilaginosa (MTCC-1403) was added to the plates at concentrations of 10 µg, 20 µg, 30 µg, and 40 µg. After 72 h of re-incubation 25µL of MTT (5 mg/mL) was added and after 3 h of incubation, the cultivation medium with MTT was aspirated and 100µL DMSO was added to each well. The absorbance was measured at a wavelength of 560 nm. The cytotoxicity was observed in terms of % cell vivality and % MCF-7 inhibition.
Influence on functionality of food products
Preparation of confectionary products
To determine the potential of extracted pigment, it was incorporated into two different confectionery products (Hard boiled candy and jelly) at concentrations of 0.1%, 0.15%, and 0.2% (w/w). Hard boiled candy was prepared by mixing the corn syrup, water and sugar with stirring until the sugar was dissolved. The mixture was then brought to boiling followed by further heating without stirring till 150 °C. It was then allowed to stand until the bubbles settled. At this stage, the extracted pigment and flavor were added. The mixture was poured into the molds and set until hardened. For the preparation of jelly, gelatin was weighed and soaked in cold water for 20 min followed by dissolving the soaked gelatin in the boiling water. The mix was stirred after the addition of sugar and salt until they dissolved. Mango juice was added for the flavor followed by addition of extracted pigment. Hot jelly was poured into molds and refrigerated until it became firm. Afterward, it was packed in butter paper lined polypropylene material.
Antioxidant activity (Radical Scavenging Activity, RSA) by DPPH
Antioxidant activity of Candy and Jelly was determined by 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assay. The carotenoids were first extracted in 80% acidified methanol. 1 g of Candy and jelly were taken and extraction was done in 20 mL of 80% acidified (0.1%) methanolic solution. 0.1 mL of the extract was treated with 3.9 mL of 0.2 mM DPPH reagent prepared in methanol. The reaction mixture was kept at 25 °C for 30 min in dark and absorbance was recorded at 515 nm using UV–Vis spectrophotometer.
Color analysis of confectionary products
The color was measured in terms of standard L*, a*, and b* values given by the CIE using Hunterlab Spectrophotometer (Colorflex Hunter Associate Lab, Reston, Virginia USA). The Spectrophotometer uses an illuminant D65 at a 10° observer as reference. The samples were filled in the cup for uniformity at the base of the cup to be pictured accurately. Color was measured in triplicates by rotating the sample cup to three varied positions (Ghoshal et al. 2013).
Texture analysis
The texture of jelly was determined by Texture Analyser TA XT Plus Stable Microsystem, UK. Spreadability is the major parameter of texture analyzed for jelly. Texture profile of jelly was analyzed in terms of firmness (N), work of shear (N. Sec) and Stickiness (kg). The samples were equilibrated at room temperature before test.
Sensory analysis
Confectionery products containing different levels of extracted pigment were coded and evaluated on 9-point hedonic scale for degree of liking ranging from “like extremely” to “dislike extremely”. 15 Semi-trained panel members were presented with the sample in a random order and were asked to mark the appropriate category on the scale. The parameters analyzed under the organoleptic evaluation were appearance, color, taste, texture, and overall acceptability (Ghoshal et al. 2013).
Statistical analysis
The values are represented as the average of three replications and the results are presented as the mean ± standard deviation (SD). The Analysis of Variance was conducted whenever required with the statistical package for the social sciences (SPSS, Inc. USA). Different superscripts were added to the values to elaborate the differences.
Results and discussion
Pigment recovery and color profile
As reported in the earlier study about the optimization of growth parameters (Sharma and Ghoshal 2020), the highest concentration of carotenoids obtained in aerobic conditions was 819.23 µg/g as shown in Table 1. The color of the extracted pigment was measured in terms of L*, a* and b* values. A decline in the L* value was observed with increase in the carotenoid content. This value measures the intensity or brightness of the color. The L* value ranged between 2.10 and 3.90. The a* value, an indication of redness of the color, ranged from 0.24 to 1.39. An increment in the positive a* value was seen with elevation in the carotenoid concentration revealing that higher the concentration of carotenoids, more was the redness in the pigment. b* value represented the yellowness of the color and varied between 0.07 and 1.55. Since carotenoids exhibit yellow to orange colors, high positive b* values were noted at higher concentration of carotenoids as shown in Table 1; however, a* and b* together explained the color profile of extracted pigment in a better way.
Table 1.
Total carotenoids and color values of pigment extracted at different intervals
| Time (h) | Total Carotenoids (µg/g) | L* | a* | b* |
|---|---|---|---|---|
| 0 | 0.00 ± 0.00i | 3.89 ± 0.69a | 0.25 ± 0.08e | 0.07 ± 0.02e |
| 6 | 42.47 ± 6.93h | 3.90 ± 0.38a | 0.24 ± 0.20e | 0.24 ± 0.11e |
| 12 | 160.57 ± 16.18g | 3.25 ± 0.33b | 0.33 ± 0.20e | 1.19 ± 0.07bc |
| 24 | 215.19 ± 19.27f | 3.26 ± 0.34b | 0.46 ± 0.10de | 1.50 ± 0.14a |
| 36 | 327.07 ± 35.21e | 2.91 ± 0.52bc | 0.79 ± 0.11cd | 1.46 ± 0.21a |
| 48 | 509.78 ± 49.12d | 3.16 ± 0.49b | 0.88 ± 0.27bc | 1.51 ± 0.23a |
| 60 | 630.28 ± 31.66c | 2.37 ± 0.14cd | 1.05 ± 0.37abc | 1.40 ± 0.07ab |
| 72 | 764.37 ± 12.73b | 2.46 ± 0.17cd | 1.21 ± 0.16ab | 1.55 ± 0.07a |
| 84 | 803.27 ± 12.04a | 2.38 ± 0.15cd | 1.39 ± 0.11a | 1.38 ± 0.13b |
| 96 | 813.44 ± 10.37a | 2.17 ± 0.11d | 1.17 ± 0.37abc | 1.00 ± 0.20cd |
| 108 | 813.79 ± 6.08a | 2.36 ± 0.17cd | 1.28 ± 0.23a | 0.98 ± 0.04 cd |
| 120 | 819.23 ± 8.52a | 2.10 ± 0.13d | 1.36 ± 0.08a | 0.93 ± 0.07d |
Results are represented as mean ± standard deviation
*Means with different superscripts in a column differ significantly (p < 0.05)
Wavelength scan of extracted pigment
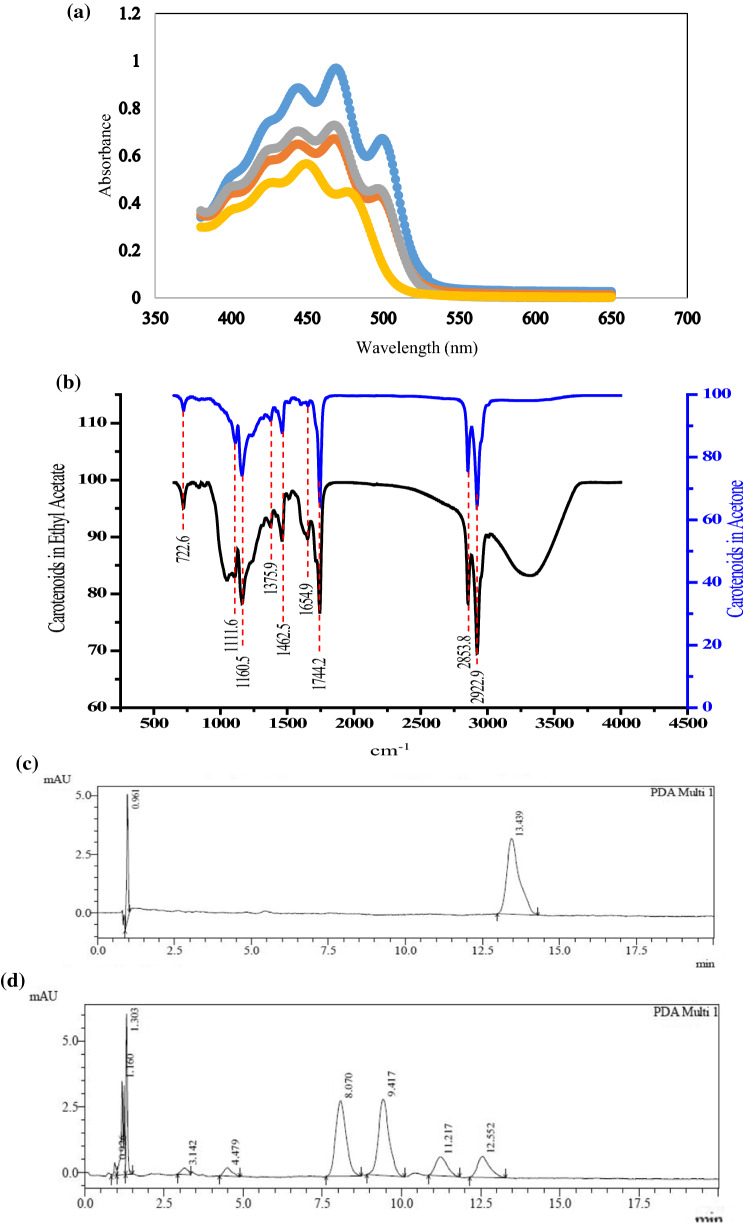
The major sharp peaks in the target region were observed at 450 nm and 480–490 nm. Moliné et al. (2012) also found similar spectra for pigment extracted from Rhodotorul mucilaginosa and stated presence of three major carotenoids. These carotenoids depicted λmax at 450 nm (β-carotene), 484 nm (Torulene) and 490 nm (Torularhodin) as shown in Fig. 1a. Although carotenoid profile remained identical in different samples, their concentrations were found to be varying as genera Rhodotorula is able to synthesize different carotenoids inclding β-carotene, torulene, and torularhodin which are synthesized via the Mevalonate Pathway (MVA) (Disch and Rohmer 1998). Initially, transformation of mevalonic acid into isopentenyl pyrophosphate units occurs and later condensation follows to form phytoene. Successful transformation of phytoene results in the synthesis of different carotenoids. However, the proportion of these carotenoids depends upon the species of the Rhodotorula explored and the fermentation conditions (Buzzini et al. 2007).
Fig. 1.
a Absorbance spectrum of extracted pigment at different concentrations. b FTIR spectrum of extracted pigment. c Chromatogram of standard β-carotene. d Chromatogram of extracted pigment
Fourier transform infrared (FTIR) spectroscopy
FTIR spectroscopy is based on the vibrational excitation of bonds by the effect of absorption of infrared light energy. Functional groups can be associated with a characteristic infrared absorption band resulting in their fundamental vibrations. Different bands and corresponding functional groups present in extracted pigment and standard β-carotene are shown in Table 2. The band present at 2972.4 cm−1 shows the common asymmetric and symmetric stretching modes of C–H groups. This generally lies in the range of 2900–3050 cm−1. The similar peak of C–H stretching was also found in extracted pigment as shown in Fig. 1b. According to Moh et al. (1999), the sharp band present at 1455 cm−1 in standard β-carotene was due to asymmetric deformation mode and another peak at 1373 cm−1 was representing symmetric deformation mode of C–H group. Two significant peaks at 956 cm−1 and 727 cm−1 in the lower frequency range corresponded to trans-conjugated alkenes and CH2 group in rocking mode respectively. The absorption bands at 1462.5–1447.6 cm−1 in extracted color arose from asymmetric deformation mode. The peaks at 967 cm−1 and 897 cm−1 in the spectrum illustrated the presence of =C–H and –C–H. Guillen and Cabo (1997) reported critical peaks at 2922 cm−1 and 2862 cm−1 for standard β-carotene representing asymmetric and symmetric stretching vibrations of CH2 and CH3. Adhoni et al. (2018) studied FTIR peaks descriptions of standard β-carotene and fungal pigment and stated that peak at 1459.6 cm−1 was due to C–H bending of alkane group. Such resembling peak was seen in the extracted pigment at 1462.5 cm−1. The intensity at particular peak is represented as weak, medium, strong and very strong. It illustrates the power of the mode of the functional group at that characteristic peak. For instance, at 1744 cm−1, the intensity of the C=O stretching is very strong while at 722.6 cm−1, the rocking mode of –CH2 is weak and the results are resemblance to study of Kaur et al. (2019).
Table 2.
FTIR peaks (cm−1) of standard β-carotene and extracted pigment
| Extracted pigment | Standard β-carotene | Intensity | Functional group | Mode |
|---|---|---|---|---|
| 2922.9 | 2972.4 | m-s | –CH3,–CH2 | C–H Stretching |
| 2853.8 | 2874.9 | m-s | –CH3,–CH | C–H Stretching |
| 1744.2 | 1717.4 | vs | –C=O | C=O Stretching |
| 1654.9 | 1717.4 | vs | –C=O | C=O Stretching |
| 1462.5 | 1447.6 | m | –CH2,–CH3 | Deformation |
| 1375.9 | 1362.4 | m | CH3 | Deformation |
| 1160.5 | 1174.6 | s | C–O | Stretching |
| 1111.6 | 1070.6 | m | C–C–C | Bending |
| 722.6 | 967.4 | w | –CH2 | Rocking |
High-performance liquid chromatography (HPLC)
It is a well-known fact that properties of the stationary phase significantly influence the separation of carotenoids during High-Performance Liquid Chromatography. Two remarkable peaks were seen in the HPLC chromatogram of standard β-carotene at a retention time of 0.961 min and 13.439 min (Fig. 1c). In comparison, the extracted pigment HPLC chromatogram illustrated 9 major peaks out of which two were in fair resemblance to standard β-carotene at 0.926 min and 12.552 min (Fig. 1d). Presently, most of the carotenoid separations are carried out using isocratic elution of short times of less than 20 min with reverse phase columns. Non-polar compounds are retarded by stationary phase and are eluted later. This effect is due to varied interaction of C18-coated silica with analytes of different polarity with the non-polar stationary phase. In such columns, the polar compounds elute first followed by non-polar compounds such as carotenes. Non-polar compounds have higher affinity towards stationary phase. Kaur et al. (2019) also characterized pigment extracted from Blakeslea trispora and reported similar elution time for β-carotene.
Fluorescence spectroscopy
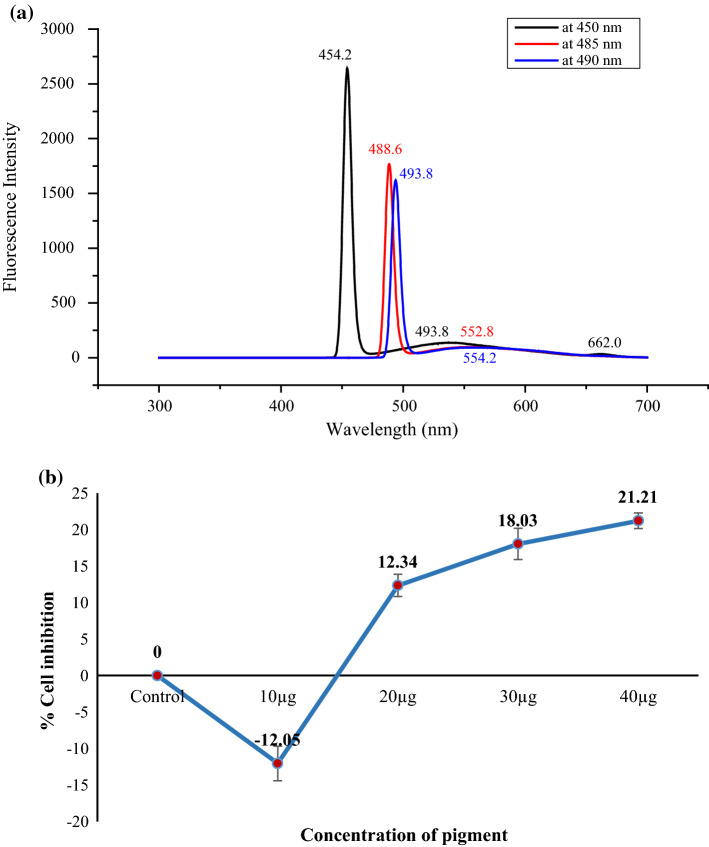
The inherent sensitivity and accuracy of fluorescence spectroscopy is an excellent alternative to obtain bands for fluorescence of carotenoids from the spectral origin. Carotenoids exhibit fluorescence and show emission spectrum at excitation wavelengths corresponding to the λmax of the UV–visible spectroscopy. The fluorescence emission graph of extracted pigment at excitation wavelengths of 450 nm, 485 nm, and 490 nm is given in the Fig. 2a. At 450 nm of excitation wavelength, a very sharp emission peak at 454.2 nm was observed followed by relatively smaller peaks at 493.8 and 662 nm depicting the presence of different compounds in the pigment which may include carotenoids or contaminants. At excitation wavelength of 485 nm and 490 nm, major emission peaks were obtained at 488–494 and 553–554 nm which may be attributed to meddling of carotenoids compounds. Despite of the fact that carotenoids do not exhibit fluorescence, weak emission spectrum around 450–550 nm was noted in the extracted pigment which is also supported by the findings of Zaghdoudi et al. (2017). Kleinegris et al. (2010) also studied emission graph of carotenoids and suggested that peak near 560 nm are most likely to be originated from β-carotene as it emits green fluorescence. Moreover, with change in the excitation wavelength, a minor shift in the emission wavelengths was also observed arising due to interference or noisiness of other similar compounds in minor concentration. Relative high intensities of emission peaks may be correlated to higher quantum yield of the respective contaminating compounds (Kleinegris et al. 2010). Few studies have reported that weak emission spectrum of carotenoids may be ascribed to the symmetrical structure of tetraterpene units formed as a result of conjugation of two geranyl geranyl di-phosphate molecules which is a basic structural characteristic of various carotenoids (Schoefs 2002).
Fig. 2.
a Emission spectrum of carotenoids in the extracted pigment at three varied excitation wavelengths (450 nm, 485 nm and 490 nm) and b MCF-7 cells inhibition by extracted pigment after 72 h
Anti-carcinogenic activity of extracted pigment
Carotenoids are bioactive compounds which possess anti-proliferative potential against several destructive cells due to their pro-oxidant ability to quench singlet oxygen molecules and to scavenge reactive oxygen species and reactive nitrogen species. The pigment extracted from R mucilaginosa was tested for its anti-carcinogenic activity against human tumor cells MCF-7 (breast cancer) cells. As shown in Fig. 2b, the extracted pigment containing β-carotene, torularhodin, and torulene was inoculated at four different concentrations (10 µg, 20 µg, 30 µg, and 40 µg) against MCF-7 cells. The results revealed that pigment was found to inhibit the viability of cancer cells. Initially, at 10 µg of extracted pigment, an increment of 12.05% in the viability of the cells was observed which later decreased significantly by 12.34% at 20 µg of pigment followed by 21.21% cell inhibition at 40 µg of pigment (Fig. 2b). Fouché et al. (2008) have developed an intensity scale for evaluation of the cytotoxicity of tested samples of β-carotene. According to that scale, pigment from R. mucilaginosa fell under the category of little activity at 40 µg of the sample. Further increase in this concentration may reach moderate activity against tumor cells. Andrade et al. (2016) investigated cytotoxicity of β-carotene by Rhodotorula glutinis against MCF-7 (breast cancer), HL-60 (promyelocytic leukemia) and J774.A1 (healthy cells of macrophages) and explained that % cell inhibition ranged between 21.72 and 61.17% against three tumor cells. Thus, the pigment extracted from R mucilaginosa was found to be useful and effective against cancer cells.
Bio-functional profile of confectionary products
Hard-boiled confectionary candy is characterized by the very lower moisture content of nearly 2–3%. Most of the natural pigments are found to be stable at a very low water activity of candies. However, thermal stability is still a major concern since color is added at 110–140 °C. With an increase in the level of color incorporation, there was rise in carotenoid content and antioxidant activity of resultant products as shown in Table 3. The carotenoid concentration of candies increased from 11.75 µg/g at 0.10% to 23.93 µg/g at 0.20% at p < 0.05. Similarly, the % radical scavenging activity ranged between 10.83 and 60.99%. The high correlation between carotenoid content and antioxidant activity is attributed to the ability of the carotenoid compounds to scavenge singlet molecular oxygen (1O2) and peroxyl radicals. Among several categories of carotenoids, the most efficient quenchers of singlet oxygen radicals are xanthophylls and carotenes. The potential of carotenoids to quench free radicals is dependent on the number of conjugated bonds which determines their lower triplet energy level.
Table 3.
Bioactive profile, color description and overall acceptability of pigmented confectionary products
| Color (%) | Carotenoids (µg/g) | RSA (%) | L* | a* | b* | Overall acceptability |
|---|---|---|---|---|---|---|
| Candy | ||||||
| 0.00 | 0.00 ± 0.00d | 10.83 ± 0.55d | 38.14 ± 0.96a | 0.36 ± 0.07d | 15.97 ± 0.59a | 7.6 ± 1.19a |
| 0.10 | 11.75 ± 0.25c | 42.49 ± 1.24c | 23.31 ± 0.31b | 8.99 ± 0.62c | 17.66 ± 0.87b | 7.9 ± 0.25a |
| 0.15 | 18.43 ± 0.22b | 55.95 ± 1.77b | 17.42 ± 1.05c | 15.96 ± 0.53b | 28.09 ± 0.83c | 7.4 ± 0.49a |
| 0.20 | 23.93 ± 0.37a | 60.99 ± 2.51a | 14.93 ± 0.29d | 17.98 ± 0.66a | 30.10 ± 0.62d | 6.2 ± 0.14b |
| Jelly | ||||||
| 0.00 | 20.27 ± 0.86d | 42.80 ± 2.75d | 37.88 ± 1.23a | 7.61 ± 0.41a | 43.56 ± 1.17a | 8.3 ± 0.46a |
| 0.10 | 30.99 ± 1.54c | 60.95 ± 2.04c | 28.76 ± 1.52b | 11.79 ± 0.29b | 41.70 ± 1.78a | 8.0 ± 0.64a |
| 0.15 | 35.59 ± 1.73b | 74.43 ± 2.04b | 27.43 ± 0.92b | 12.04 ± 0.18b | 36.99 ± 0.50b | 7.5 ± 0.51ab |
| 0.20 | 39.50 ± 1.32a | 86.19 ± 1.95a | 18.90 ± 0.70c | 15.83 ± 0.52c | 31.02 ± 0.85c | 6.9 ± 0.75b |
Results are represented as mean ± standard deviation
*Means with different superscripts in a column differ significantly (p < 0.05)
The effect of the addition of color at different levels was clearly visible from the appearance properties of candy. Higher the concentration of pigment, more was the intensity of the color. The L* value ranged between 14.93 and 38.14 while a* was found to be varying between 0.36 and 17.98 and b*, indicating the yellowness of color, increased from 15.97 to 30.10 at p < 0.05. Addition of pigment to candies resulted in opaque cloudy appearance. This effect was due to emulsification which occurred due to the lipophilic nature of the pigment suggesting that if transparency is targeted in the final product, the use of water-soluble pigments is appreciated.
The highest score for appearance was obtained by candy with 0.1% pigment. Further increase in color made the candy more intense and cloudier. The color of candy at 0.2% pigment was brownish which exhibited a lowest sensory score. With respect to taste, the candies with 0.2% color were slightly bitter in comparison to others at lower concentration. Johnson and Clydesdale (1982) also demonstrated the effect of food color on taste perceptions and found that the addition of color intensified the flavor. The darker colored samples were 2–10% different from light-colored samples in terms of taste. However, Maga (1974) explained the correlation between redness and sweetness levels in natural foods. Zellner et al. (1991) also reported that food colors influence perceived flavor identity. They stated that panelists were able to correctly identify odors more rapidly than when uncolored. Thus, the candy with 0.1% exhibited the highest score for overall acceptability.
Confectionary gels contain high sugar components of sucrose and glucose syrup, along with gelling components like gelatin, pectin or starch with added flavors and colors. Addition of color to jelly imparted a substantial effect on consumer acceptability. The results revealed that there was a linear relation between Carotenoids and antioxidant activity with an increase in concentration of the color. The addition of color at levels resulted in an escalation in carotenoids content from 20.27 µg/g at 0.0% to 39.5 µg/g at 0.20%.
Moreover, the antioxidant activity for jelly increased from 42.80 to 86.19% at levels of 0.0 to 0.20% color addition. The carotenoid content and respective antioxidant activity of control sample was due to the addition of natural mango juice as a flavoring agent. The higher antioxidant activity was due to the ability of carotenoids to quench free radicals. Also, L* values ranged from 37.88 for control to 18.90 for jelly containing 0.20% pigment. a* also increased from 7.61 to 15.83 as it expresses the redness of the sample. However, a decrease in the yellowness i.e. b* was also observed with increase in color concentration. The value of b* varied between 31.02 and 43.56 at p < 0.05. The texture of jelly was influenced with incorporation of extracted pigment as significant change was observed in the firmness of jelly with variation in the level of color addition. Control sample containing no added color exhibited firmness of 6.70 N which decreased to 4.262 N at 0.1% added color. A further decrease was noted to 3.60 N at the level of 0.2% color addition to jelly as shown in Table 4. There was significant decline in the work of shear from 6.24 N.sec. to 3.35 N.sec with increment in color from 0.0 to 0.20%.
Table 4.
Effect of pigment concentration on the texture of jelly
| Color (%) | Firmness N | Work of Shear N.sec | Stickiness kg |
|---|---|---|---|
| 0.00 | 6.70 ± 0.16a | 6.24 ± 0.10a | − 0.22 ± 0.03c |
| 0.10 | 4.36 ± 0.18b | 3.05 ± 0.11b | − 0.15 ± 0.01b |
| 0.15 | 3.92 ± 0.12c | 2.57 ± 0.08c | − 0.10 ± 0.02a |
| 0.20 | 3.60 ± 0.18d | 2.35 ± 0.12d | − 0.09 ± 0.02a |
Results are represented as mean ± standard deviation
Means with different superscripts in a column differ significantly (p < 0.05)
Similar to candy, organoleptic profile was affected by pigment concentration. The highest sensory score for texture was noted 0.1% pigmented jelly and control. There was a slightly bitter after taste at a higher level of color addition. Incorporation of bioactive compounds in food is often related to increased bitterness at certain concentrations; however, reduction in off taste has been successfully achieved by techniques such as blocking, suppressing, masking and inhibiting mechanisms. Certain other approaches such as encapsulation with improved stability, bioavailability and bioactivity have been proposed. The hedonic scores for overall acceptability of jellies with different concentrations of extracted pigment are shown in Table 3. The appearance in terms of color also varied with a change in the concentration of added pigment. Jelly with 0.2% added color was brighter than at lower concentration of color. The highest sensory score for color and appearance was observed for sample containing 0.1% of added pigment. A little effect was seen on the texture perception by panelists. With increase in addition of extracted pigment to jelly, hedonic score of texture decreased from 7.4 to 6.7. The sensory scores revealed that the incorporation of color at 0.1% in jelly was most accepted with overall acceptability score of 8.0.
Conclusion
Carotenoids are not only effective radical scavengers which quench the singlet oxygen molecules reducing the oxidative stress which would otherwise be responsible for the onset of degenerative diseases but also act as Pro-vitamin A, an important nutrient for healthy vision and other metabolic processes in the body. The daily intake of carotenoids is highly significant. Production of stable carotenoids at lower costs is one of the major challenges today. Therefore, synthesis of carotenoids by biotechnological means using low-cost Agro-industrial by-products seeks to give a higher yield of carotenoids at lower processing cost with greater stability in comparison to the plant-based carotenoids. Biocolor produced from R mucilaginosa is a heat stable mixture of several carotenoids predominantly torularhodin, β-carotene, and torulene. The sensory evaluation results revealed that the addition of color at 0.1% in jelly and candy was acceptable producing overall acceptability score of 8.0. The biochemical interactions of such pigment in different food systems and their impact on bioactive and organoleptic properties need further exploration in near future.
Acknowledgement
Both the authors are thankful to PURSE II and TEQIP II & III, UGC-SAP, DR. SS. Bhatnagar University Institute of Chemical Engineering and Technology, Panjab University, Chandigarh for financial Assistance. Authors are thankful to Dr. Vandana, PGIMR to help to conduct Cell line study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adhoni SA, MeghaR S, Shivasharana CT. FTIR analysis of β-carotene produced from Chlorella vulgaris as-3 strain, a fresh water micro algae. Asian J Sci Technol. 2018;9:7610–7615. [Google Scholar]
- Andrade RFS, Lima RA, Ribeaux DR, et al. Production of β-carotene by a newly isolated Rhodotorula Glutinis UCP1555 strain and cytotoxic effect evaluation. J Chem Chem Eng. 2016;10:212–220. [Google Scholar]
- Britton G, Liaaen-Jensen S, Pfander H. Carotenoids, vol. 4: natural functions. Berlin: Springer; 2008. [Google Scholar]
- Buzzini P, Innocenti M, Turchetti B, et al. Carotenoid profiles of yeasts belonging to the genera Rhodotorula, Rhodosporidium, Sporobolomyces, and Sporidiobolus. Can J Microbiol. 2007;53:1024–1031. doi: 10.1139/W07-068. [DOI] [PubMed] [Google Scholar]
- Buzzini P, Martini A. Production of carotenoids by strains of Rhodotorula glutinis cultured in raw materials of agro-industrial origin. Bioresour Technol. 2000;71:41–44. doi: 10.1016/S0960-8524(99)00056-5. [DOI] [Google Scholar]
- Cheng Y-T, Yang C-F. Using strain Rhodotorula mucilaginosa to produce carotenoids using food wastes. J Taiwan Inst Chem Eng. 2016;61:270–275. doi: 10.1016/j.jtice.2015.12.027. [DOI] [Google Scholar]
- Chreptowicz K, Mierzejewska J, Tkáčová J, et al. Carotenoid-producing yeasts: identification and characteristics of environmental isolates with a valuable extracellular enzymatic activity. Microorganisms. 2019;7:653. doi: 10.3390/microorganisms7120653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JM, Parajo JC. Improved astaxanthin production by Xanthophyllomyces dendrorhous growing on enzymatic wood hydrolysates containing glucose and cellobiose. Food Chem. 1998;63:479–484. doi: 10.1016/S0308-8146(98)00061-2. [DOI] [Google Scholar]
- Disch A, Rohmer M. On the absence of the glyceraldehyde 3-phosphate/pyruvate pathway for isoprenoid biosynthesis in fungi and yeasts. FEMS Microbiol Lett. 1998;168:201–208. doi: 10.1111/j.1574-6968.1998.tb13274.x. [DOI] [PubMed] [Google Scholar]
- Fouché G, Cragg GM, Pillay P, et al. In vitro anticancer screening of South African plants. J Ethnopharmacol. 2008;119:455–461. doi: 10.1016/j.jep.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Frengova G, Simova E, Beshkova D. Use of whey ultrafiltrate as a substrate for production of carotenoids by the yeast Rhodotorula rubra. Appl Biochem Biotechnol. 2004;112:133–141. doi: 10.1385/ABAB:112:3:133. [DOI] [PubMed] [Google Scholar]
- Ghoshal G, Shivhare US, Banerjee UC. Effect of xylanase on quality attributes of whole-wheat bread. J Food Qual. 2013;36:172–180. doi: 10.1111/jfq.12034. [DOI] [Google Scholar]
- Guaadaoui A, Benaicha S, Elmajdoub N, et al. What is a bioactive compound? A combined definition for a preliminary consensus. Int J Nutr Food Sci. 2014;3:174–179. doi: 10.11648/j.ijnfs.20140303.16. [DOI] [Google Scholar]
- Guillen MD, Cabo N. Infrared spectroscopy in the study of edible oils and fats. J Sci Food Agric. 1997;75:1–11. doi: 10.1002/(SICI)1097-0010(199709)75:1<1::AID-JSFA842>3.0.CO;2-R. [DOI] [Google Scholar]
- Hayashi M, Matsui M, Ishii K, Kawasaki M. Genotoxicity evaluation datasheet of food additives by the MHW (1980–1998) Env Mutagen Res. 2000;22:27–44. [Google Scholar]
- Jain A, Thakur D, Ghoshal G, et al. Characterization of microcapsulated β-carotene formed by complex coacervation using casein and gum tragacanth. Int J Biol Macromol. 2016;87:101–113. doi: 10.1016/j.ijbiomac.2016.01.117. [DOI] [PubMed] [Google Scholar]
- Johnson J, Clydesdale FM. Perceived sweetness and redness in colored sucrose solutions. J Food Sci. 1982;47:747–752. doi: 10.1111/j.1365-2621.1982.tb12706.x. [DOI] [Google Scholar]
- Joshi VK, Attri D, Bala A, Bhushan S. Microbial pigments. Indian J Biotechnol. 2003;2(3):362–369. [Google Scholar]
- Kaur P, Ghoshal G, Jain A. Bio-utilization of fruits and vegetables waste to produce β-carotene in solid-state fermentation: Characterization and antioxidant activity. Process Biochem. 2019;76:155–164. doi: 10.1016/j.procbio.2018.10.007. [DOI] [Google Scholar]
- Kesava SS, An G-H, Kim C-H, et al. An industrial medium for improved production of carotenoids from a mutant strain of Phaffia rhodozyma. Bioprocess Eng. 1998;19:165–170. [Google Scholar]
- Kim S-W, Seo W-T, Park Y-H. Enhanced synthesis of trisporic acid and β-carotene production in Blakeslea trispora by addition of a non-ionic surfactant, Span 20. J Ferment Bioeng. 1997;84:330–332. doi: 10.1016/S0922-338X(97)89253-7. [DOI] [Google Scholar]
- Kleinegris DM, van Es MA, Janssen M, et al. Carotenoid fluorescence in Dunaliella salina. J Appl Phycol. 2010;22:645–649. doi: 10.1007/s10811-010-9505-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maga JA. Influence of color on taste thresholds. Chem Senses. 1974;1:115–119. doi: 10.1093/chemse/1.1.115. [DOI] [Google Scholar]
- Martin AM, Lu C, Patel TR. Growth parameters for the yeast Rhodotorula rubra grown in peat extracts. J Ferment Bioeng. 1993;76:321–325. doi: 10.1016/0922-338X(93)90202-J. [DOI] [Google Scholar]
- Moh MH, Man YC, Badlishah BS, et al. Quantitative analysis of palm carotene using Fourier transform infrared and near infrared spectroscopy. J Am Oil Chem Soc. 1999;76:249. doi: 10.1007/s11746-999-0226-9. [DOI] [Google Scholar]
- Moliné M, Libkind D, van Broock M. Production of torularhodin, torulene, and β-carotene by Rhodotorula yeasts. In: Barredo José-Luis., editor. Microbial carotenoids from fungi. Berlin: Springer; 2012. [DOI] [PubMed] [Google Scholar]
- Nancib A, Nancib N, Boubendir A, Boudrant J. The use of date waste for lactic acid production by a fed-batch culture using Lactobacillus casei subsp. rhamnosus. Braz J Microbiol. 2015;46:893–902. doi: 10.1590/S1517-838246320131067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parajó JC, Santos V, Vázquez M. Optimization of carotenoid production by Phaffia rhodozyma cells grown on xylose. Process Biochem. 1998;33:181–187. doi: 10.1016/S0032-9592(97)00045-9. [DOI] [Google Scholar]
- Polulyakh OV, Podoprigova OI, Eliseev SA, Ershov YV, Bykhovsky VY, Dmitrovski AA. Biosynthesis of torulene and torularhodin in the yeast Phaffia rhodozyma. Prikl Biokhim Microbiol. 1991;27:541–545. [Google Scholar]
- Rabeta MS, Chan S, Neda GD, et al. Anticancer effect of underutilized fruits. Int Food Res J. 2013;20:551–556. [Google Scholar]
- Schoefs B. Chlorophyll and carotenoid analysis in food products. Properties of the pigments and methods of analysis. Trends Food Sci Technol. 2002;13:361–371. doi: 10.1016/S0924-2244(02)00182-6. [DOI] [Google Scholar]
- Sharma R, Ghoshal G. Optimization of carotenoids production by Rhodotorula mucilaginosa (MTCC-1403) using agro-industrial waste in bioreactor: a statistical approach. Biotechnol Rep. 2020;25:e00407. doi: 10.1016/j.btre.2019.e00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuli HS, Chaudhary P, Beniwal V, Sharma AK. Microbial pigments as natural color sources: current trends and future perspectives. J Food Sci Technol. 2015;52:4669–4678. doi: 10.1007/s13197-014-1601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghdoudi K, Ngomo O, Vanderesse R, et al. Extraction, identification and photo-physical characterization of persimmon (Diospyros kaki L.) carotenoids. Foods. 2017;6(1):4. doi: 10.3390/foods6010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellner DA, Bartoli AM, Eckard R. Influence of color on odor identification and liking ratings. Am J Psychol. 1991;104:547–561. doi: 10.2307/1422940. [DOI] [PubMed] [Google Scholar]
- ZHANG WSZX, Kai HL. Effects of some additives on the growth and carotenoids content of Rhodotorula. Food Sci Technol. 2001;2:6. [Google Scholar]