Abstract
We provide here a general view on the interactions of surfactants with viruses, with a particular emphasis on how such interactions can be controlled and employed for inhibiting the infectivity of enveloped viruses, including coronaviruses. The aim is to provide to interested scientists from different fields, including chemistry, physics, biochemistry, and medicine, an overview of the basic properties of surfactants and (corona)viruses, which are relevant to understanding the interactions between the two. Various types of interactions between surfactant and virus are important, and they act on different components of a virus such as the lipid envelope, membrane (envelope) proteins and nucleocapsid proteins. Accordingly, this cannot be a detailed account of all relevant aspects but instead a summary that bridges between the different disciplines. We describe concepts and cover a selection of the relevant literature as an incentive for diving deeper into the relevant material. Our focus is on more recent developments around the COVID-19 pandemic caused by SARS-CoV-2, applications of surfactants against the virus, and on the potential future use of surfactants for pandemic relief. We also cover the most important aspects of the historical development of using surfactants in combatting virus infections. We conclude that surfactants are already playing very important roles in various directions of defence against viruses, either directly, as in disinfection, or as carrier components of drug delivery systems for prophylaxis or treatment. By designing tailor-made surfactants, and consequently, advanced formulations, one can expect more and more effective use of surfactants, either directly as antiviral compounds or as part of more complex formulations.
Keywords: Surfactant, Virus inactivation, Disinfection, Enveloped viruses, Lipid bilayers
Abbreviations: AFM, atomic force microscopy; BVDV, Bovine Viral Diarrhea Virus; C12E8, dodecyloctaglycol; cac, critical aggregate concentration; cmc, critical micelle concentration; CPyC, cetylpyridinium chloride; DSPC, 1,2-distearoyl-sn-glycero-3-phosphocholine; Flu, influenza virus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; ITC, isothermal titration calorimetry; Ld, liquid-disordered; Lo, liquid-ordered; p, packing parameter; PA, phosphatidic acid (anionic); PC, phosphatidylcholine (zwitterionic); PE, phosphatidylethanolamine (zwitterionic); PI, phosphatidylinositol (anionic); POPC, 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; PS, phosphatidylserine (anionic); QUAT, quaternary alkyl ammonium; RNP, ribonucleoprotein particle; SAXS, small-angle X-ray scattering; SDS, sodium dodecyl sulphate; TBP, tri-n-butyl phosphate; TEM, transmission electron microscopy
Introduction
The COVID-19 pandemic caused by SARS-CoV-2 that has been ongoing for more than 18 months has drawn broader attention to viruses and initiated widespread academic research activities with the goal of controlling or suppressing the virus and the disease. From a physicochemical point of view, viruses are complex, self-assembled colloids built from genetic material, either DNA or RNA, a proteinaceous nucleocapsid, and in some instances, a lipid/glycoprotein envelope (enveloped viruses). A prominent example of enveloped viruses are coronaviruses [1]. Of course, there is a high interest in suppressing virus activity and spread, which limit negative consequences on human health. When looking at the scientific questions involved, one can justifiably argue that control of SARS-CoV-2 is, at least to a certain extent, a colloidal question [2]. In this context, the topic of how nanotechnology can be employed for stopping enveloped viruses has been reviewed recently [3], and the same applies to the antiviral properties of polymers [4].
Another way of interfering with virus activity is by employing surfactants, as they are known to interact with DNA/RNA, proteins, and lipids, i.e., with all the building blocks of a virus. Of course, there have been many studies regarding the effect of individual surfactants (or groups thereof) on virus activity, especially in the context of disinfection [5, 6, 7], but a further reaching general discussion is largely missing. In addition, one has here a field where one has to bridge between the disciplines focussing on the virus, virology and biochemistry, and the ones focussing on surfactants, colloid science and its physical chemistry. These disciplines often operate quite independently, and a closer cooperation certainly is of mutual benefit.
The aim of this review is to provide a general overview of the prevailing conditions when we consider the interactions of surfactants with viruses, mainly with the intent of inactivating the viruses’ biochemical activity, with an emphasis on the current developments regarding coronaviruses, which are rapidly evolving at the moment. This will first be done by giving a concise description of biological membranes, including the embedded proteins, and their presence in enveloped viruses, and a short description of virus structure and properties. We describe in some detail the lipid bilayer composition and its properties and how that is related to various organelles within a cell, which in turn affects the lipid composition of a virus envelope. Next, we will recall some basic facts and properties of surfactants that are relevant to their ability to interact with components of self-assembled colloids, including viruses. In the next steps, we then focus on surfactant interactions with model lipid membranes and different types of viral proteins separately before reporting on recent work on the interaction of surfactants with real viruses. We do so with a focus on enveloped viruses, including SARS-CoV-2, with the aim of reducing or eliminating virus infectivity. Of course, for enveloped viruses, the surfactant primarily interacts with the protein-studded lipid envelope.
This review combines the complex and comprehensive view from a virology perspective with the simplifying approach from the physics and physical chemistry angle. By doing so, our aim is to relate general physicochemical principles to the actual behaviour of surfactants in a complex situation, which is the case if surfactants are mixed with viruses. This approach should deliver general guidelines for how to best apply surfactants in the context of inhibiting virus infectivity and broaden the view with respect to the aspects relevant in such undertakings.
Biological membranes
As the focus of this review is on enveloped viruses, we will first describe their distinctive feature, the lipid membrane that constitutes the envelope. It is central to the function of this group of viruses, and we will recall some of the basic properties of lipid membranes before describing in more detail their composition and properties in viruses, which is a central aspect to be considered in any approach aiming at disabling virus function by destroying membrane integrity. The lipid envelope is usually the most vulnerable part of a virus particle, and hence, enveloped viruses are easier to inactivate when compared with nonenveloped viruses.
Biophysical properties of biological membranes
Biological membranes are complex structures that consist of many different lipids (phospholipids, sphingolipids, cholesterol) and proteins. Depending on the temperature, biomembranes occur in different phases: At low temperatures, the lipids form a solid gel, where the order of their acyl chains is high and lateral movement is low. Upon warming, the bilayer transitions into a fluid, ‘liquid-disordered’ (Ld) phase, where individual lipid molecules diffuse in the membrane’s plane, rotate along their vertical axes and show considerable flexing and bending of their fatty acid tails. The phase transition temperature of membranes depends on the length of the lipids’ acyl chain and their number of double bonds. Shorter and unsaturated acyl chains have reduced possibilities to interact with each other, and as a result, lower the phase transition temperature. Another important regulator of lipid organisation is cholesterol, which reduces the motion of lipids, and thus, the fluidity of the bilayer and also broadens the temperature range in which phase transition occurs [8].
Cholesterol is also essential for the formation of ‘membrane rafts’, dynamic assemblies of cholesterol, sphingolipids, and phospholipids containing mainly saturated fatty acids, which are segregated from the bulk phase of the membrane. Rafts form a ‘liquid-ordered’ (Lo) phase, in which acyl chains are restricted in mobility, but lipids are still able to diffuse and rotate. Rafts are kept together by hydrophilic and hydrophobic forces. Due to the rigid structure of the sterol ring, cholesterol molecules can be densely packed with saturated fatty acid chains of phospholipids, which restricts their motion. Sphingolipids, the only lipid possessing both hydrogen bond donor and acceptor groups, can form weak interactions with each other and with the hydroxyl group of cholesterol. The spatial separation of Lo and Ld domains causes a hydrophobic mismatch and a height difference between the two membrane phases, leading to the formation of a ‘line tension’ at their interface, which is conceptually comparable to surface tension in a three-dimensional system. Line tension results in the formation of a curved raft phase due to the propensity of the system to minimize the contact area between both phases [9]. Rafts are used by several viruses as a platform for the assembly of their envelope proteins and the subsequent budding event (see below).
Phase separation can be visualised and characterised in model membranes composed of only three different lipids [10]. Such phase separation can experimentally be seen by means of super-resolution optical microscopy [11], even in living cells [12], or atomic force microscopy (AFM) [13]. However, membranes consist of a much more complex lipid mixture and proteins, and no large-scale, long-lasting phase separation can be observed by conventional light microscopy. Instead, highly dynamic (millisecond range) and very small (10–200 nm) lipid assemblies have been observed using biophysical methods that allow high temporal and spatial resolution [14, 15, 16, 17]. Under certain conditions, such as upon ligand binding and receptor oligomerisation, ‘resting state’ rafts can be coalesced and stabilized to fulfil a biological function, such as facilitating signal transduction [14, 15, 16, 17] [14, 15, 16, 17] [14, 15, 16, 17]. The assembly and budding of many viruses, including those of human immunodeficiency and influenza viruses, are dependent on the formation of functionalised raft domains. In this context, rafts provide a scaffold that results in targeting and concentrating on structural virus proteins and facilitating their interactions, while cellular proteins are largely excluded [18].
Interaction of lipids with proteins
Since both proteins and lipids are mobile within lipid membranes, the membrane-contacting region of a protein constantly interacts with various lipids. These interactions are highly dynamic; a boundary lipid molecule typically resides for less than 1 μs at the protein’s surface before it is replaced by another lipid molecule; however, certain lipids bind with higher specificity to recognition motifs of a protein in a manner consistent with a typical ligand or cofactor, which, in turn, can affect the activity of the protein bind. A prime example is cholesterol-binding to seven transmembrane receptors, which was discovered by crystallography and allowed the identification of protein consensus motifs for cholesterol binding [19]. A similar binding site for cholesterol is present in the outer part of the transmembrane region of the hemagglutinin of some influenza virus strains [20], of the glycoprotein of the Ebola virus [21] and the spike glycoprotein (S) of SARS-CoV-2 [22]. The membrane proteins of the Zika virus (and possibly other flaviviruses) contain two binding sites for a Y-shaped lipid [23]. In both viruses, mutagenesis of the amino acids involved in lipid-binding greatly reduced virus growth in cell culture [20, 21, 22, 23∗]. Furthermore, lipids may bind to regions of a protein that do not contact the membrane. Cryo-EM of the SARS-CoV-2 S revealed that its receptor-binding domains tightly bind the unsaturated fatty acid, linoleic acid, an interaction that stabilizes a locked conformation, resulting in reduced binding to its receptor [24]. In addition, even nonenveloped viruses might contain lipids. For example, more than 220 lipid species could be extracted from purified iridoviruses that do not have an envelope and identified by mass spectrometry [25].
The shape of lipids affects membrane curvature
A flat membrane must be transformed into a hollow sphere to endow a new virus particle with a lipid envelope, while the opposite shape change occurs during virus entry by membrane fusion. Thus, a force must act on the bilayer to cause curvature, which is provided by proteins that push or pull on the membrane. However, lipids also contribute to these shape alterations since individual lipids differ in intrinsic curvature. Most phospholipids have a cylindrical shape, i.e., the hydrophilic head group and the hydrophobic tail are about the same size, and their side-by-side arrangement prefers to generate flat membranes. Some lipids, such as cholesterol, are cone-shaped, composed of a small head group and a large tail. Other lipids, for example, lysophospholipids, are shaped like an inverted cone having a large head group and a small tail. Lipids with an intrinsic curvature positively or negatively affect certain stages of bud formation. Inverted cones present in the outer leaflet and cones in the inner leaflet facilitate a positive (outward facing) curvature of a flat membrane. Likewise, changes in curvature also occur during membrane fusion, and certain lipids are known to facilitate or hamper this process [26]. Similarly, one can expect surfactants with their different spontaneous curvature to have such effects that suppress membrane fusion and/or virus budding.
It can be noted that the geometric effects just described for the packing and curving of lipid bilayers typically are described in surfactant science in a more quantitative fashion by the dimensionless packing parameter p that is defined as the ratio between the volume of the hydrophobic part of the amphiphile va, by the product of the head group area ah (the area required by the molecule at the amphiphilic interface) and the stretched length L of the hydrophobic part, i.e., p = va/(ah·L) [27]. According to a simple geometric consideration one then will form spherical aggregates for: p < 1/3, cylindrical aggregates for: 1/3 < p < 1/2, and bilayers for: 1/2 < p < 1.
Intracellular distribution of lipids
The main lipid biosynthetic organelle is the endoplasmic reticulum (ER), which produces phospholipids, cholesterol and ceramide, the precursor for complex glycosphingolipids. However, the ER displays only low concentrations of sterols since they are rapidly transported to the Golgi complex and to the plasma membrane. As a result, the molar ratio of cholesterol to phospholipids is only around 0.15 in the ER, but successively increases throughout the Golgi cisternae, and finally reaches values of 1.0 at the plasma membrane. This distribution is consistent with the function of these organelles: insertion and transport of newly synthesized lipids and proteins in the ER and resistance against mechanical stress of the plasma membrane. Minor differences also exist for the distribution of individual phospholipids along the membranes of the exocytotic pathway, i.e. phosphatidylserine (PS) increases and phosphatidylinositol (PI), phosphatidylethanolamine (PE) and phosphatidylcholine (PC) decrease. Likewise, the concentration of glycosphingolipids, which are synthesized from ceramide and then heterogeneously glycosylated in the Golgi complex, also increases along the exocytotic pathway. In polarized cells, they are exclusively transported to the apical membrane, which is separated by a diffusion barrier from the basolateral plasma membrane.
During intracellular transport, there is another reason why an uneven distribution of lipids builds up. Whereas all lipids are symmetrically distributed between the two leaflets of the ER bilayer, the Golgi and the plasma membranes display an asymmetric lipid distribution: Glycolipids are exclusively present on the luminal side, whereas PE, PS and PI are enriched in the cytosolic leaflet. Once established, the asymmetric distribution of lipids is stable, and the flip-flop of individual molecules across the membrane occurs on the time scale of days [28,29], which is surprisingly slow for the movement across such a small distance.
The basic architecture of enveloped viruses
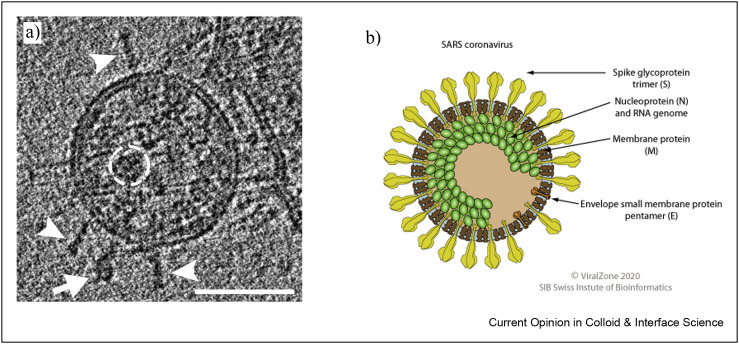
A virus is a self-assembled system containing nucleic acid (RNA or DNA) and proteins, where positively charged proteins form a shell, the nucleocapsid, around the condensed RNA/DNA molecules [30]. Nucleocapsids can adopt an icosahedral or rod-like (helical) shape, and the latter exemplified by TMV (tobacco mosaic virus), and were one of the first important structures characterized by electron microscopy in the 1930s. In many cases, the basic structure is complemented by lipids that form an envelope around this basic assembly. Accordingly, one distinguishes nonenveloped and enveloped viruses. Coronaviruses are RNA viruses belonging to the latter group with a typical spherical appearance, and the SARS-CoV-2 particle has a mean outer diameter of 91 ± 11 nm (Figure 1 ) [31].
Figure 1.
a) Cryo-TEM image of SARS-CoV-2 virions. Readily visible are the S trimers protruding from the virion surface (scale bar: 50 nm) [40]. Similar images can also be seen in Ref. [40]. b) Sketch of a SARS-CoV.
Most suited to gain detailed structural information regarding the mesoscopic structure of most viruses is cryo-electron microscopy (cryo-EM) [31] or cryo-electron tomography (cryo-ET) [32], techniques that even allow deduction of structural details, including those for the spike [33]. Cyro-EM and cryo-ET are particularly suited here as the vitrified film of an aqueous solution typically has a thickness of 100–300 nm, and therefore, is perfectly suited size-wise to host the virus particles and study them.
Proteins of coronaviruses
Of course, the main site of surfactant interaction with coronaviruses (and this applies to any virus) are the proteins contained in the virus envelope.
The most frequently encountered protein in the coronavirus envelope is the M protein (1000–2000 molecules per particle [34]) that has a short ectodomain, three transmembrane regions and a longer cytoplasmic tail. It forms oligomers within the virus membrane and is controlling membrane curvature by its conformers [35]. Another abundant structural protein is the nucleocapsid protein N (1000–2000 molecules per particle), which forms complexes with parts of genomic RNA, called ribonucleoparticles (RNPs), with a bead-on-a-string morphology. One virus particle contains around 25 RNPs, and some of them are attached to the inner side of the envelope [36]. The most prominent protein of coronaviruses, not in abundance but in terms of protruding from the membrane, is the S protein (around 25+-10 trimers per particle) that is the sole structural component of the viral spike [37], is central for receptor recognition, cellular attachment and entry. As this is the central point of infection, most attention is focused on blocking the activity of S proteins [38], which are well visible in Figure 1a. Sixty-six carbohydrates are covalently attached to one S trimer, and each carbohydrate chain is composed of 7–12 monosaccharides. As a result, most of the surface of S is covered with hydrophilic sugar moieties. The stalk region of the spike contains three flexible hinges, which allows the head domain to bend relative to the membrane to scan the cell surface for putative receptors [39]. Only around 20 copies of the small and hydrophobic E protein are present in the membrane, where they form an ion channel facilitating virus entry.
Lipid composition of enveloped viruses
Enveloped viruses assemble at and bud from either the plasma membrane or from membranes of the exocytic pathway, which exhibit different lipid compositions. Since a viral capsid is coated with lipids when it buds through the bilayer, it was suggested that the lipid composition of the viral envelope is mostly determined by the host membrane [41]. This has now been most convincingly shown for viruses from four different families that assemble at the plasma membrane. Quantitative mass spectrometry revealed that the lipid composition (~160 lipid species) of Semliki Forest Virus (a togavirus) and Vesicular Stomatitis Virus (a rhabdovirus) are indistinguishable if grown in the same cell type, and only slight differences were detected between the viral lipid composition and that of the plasma membrane [42]. Viruses supposed to bud through raft-domains, such as influenza viruses and HIV, are slightly enriched in cholesterol and sphingolipids compared to the apical plasma membrane (influenza) or are substantially enriched in the rare sphingolipid dihydrosphingomyelin (HIV) [43,44].
For viruses that bud from intracellular membranes, only two sophisticated lipid analyses are available [45,46]. Strikingly, cholesterol ester, a storage form of cholesterol that is not a component of cellular membranes, is the major component of the envelope of Hepatitis C Virus (HCV), followed by cholesterol, although the virus buds through the ER membrane, which is low in cholesterol. The unique lipid composition is a result of the unusual egress pathway of HCV that coopts very-low-density lipoprotein (VLDL) particles, which are secreted from liver cells to supply the body via the bloodstream with lipids [45]. However, cholesterol and sphingolipids are also prominent in the envelope of Bovine Viral Diarrhea Virus (BVDV), another member of the Flaviviridae, and its lipid composition is very similar to that of the influenza virus. It was, therefore, suggested that BVDV either buds through (hypothetical) ER microdomains already enriched in cholesterol and sphingolipids or recruits these lipids to the budding site [46].
No detailed lipidomic analysis is available for other viruses that bud through intracellular membranes, but sphingomyelin, a ‘raft-lipid’ is enriched relative to the lipid content of the whole cell in the envelope of both Equine Arteritis Virus, a member of the Arteriviridae that are closely related to Coronaviridae, and mouse hepatitis virus, a coronavirus [47].
In summary, viruses tend to integrate lipids into their envelope that increase the mechanical stiffness of their membranes [48]. In fact, the lipid composition of enveloped viruses is remarkably similar, even if they derive from different cellular membranes. The main constituent is always cholesterol (37–52 mol%), followed by phospholipids (30–37%) and sphingolipids (18–20%). In contrast to cholesterol and sphingolipids, the content of the individual phospholipid species roughly corresponds to the membrane from which the virus is derived. The main phospholipid in both HCV and BVDV is PC, which is also predominant in the ER membrane, whereas viruses that bud at the plasma membrane contain mainly PS that is enriched in the plasma membrane relative to the ER [41, 42, 43, 44∗, 45, 46, 47]. Furthermore, the surface proteins of the Dengue virus that do not form spike-like protrusions but lie flat on the membrane in an icosahedral-like symmetry interact extensively with the lipids conferring ‘‘raft-like’’ robustness even on a cholesterol-poor membrane [49].
It might be noted that, for instance, the influenza lipid envelope is softer than expected for a gel phase bilayer and its stiffness changes only rather little with increasing temperature [50]. Certainly also the proteins contained in the lipid envelope play a relevant role in determining the membrane stiffness. For instance, AFM experiments on the lipid envelope of the influenza virus showed double the stiffness compared to just the pure phosphatidylcholine liposomes, an effect attributed to the membrane-attached protein components [51].
There are several requirements on the biophysical properties of a viral envelope for optimal transmissibility: The viral pre-envelope must be fluid at body temperature to allow the budding of new virus particles. Once the virus is exposed to the environment, the membrane should solidify (gel state) to protect virions against damage during transmission between organisms. After entry into a new host, the viral membrane must liquefy again to allow infection by fusion of its envelope with cellular membranes. It was shown for the influenza virus that the temperature profile of its phase transitions roughly fulfils these requirements [52]. Above 42 °C, the virus membrane is uniformly liquid, but below 42 °C, ordered domains appear that coexist with liquid phases. The fraction of the latter decreases with decreasing temperature until at ~4 °C, the whole membrane is solid. The presence of cholesterol was critical for the formation of these phases.
Virus structure from a simple colloid perspective
Despite the fact that the detailed build-up of a virus in terms of molecular components is very complex and normally not fully known for a given virus, its description as a colloidal object may be much too simplified. A very comprehensive review regarding growth, form, and properties, as seen from the physics point of view, has recently been given [53]. As a virus must contain highly charged DNA or RNA, compacted by (at least locally) cationic proteins, the electrostatics in such systems are important and an excellent discussion of the relevant aspects has been given some years ago [54]. However, electrostatics is not only important for understanding the properties of viruses but is also the major driving force for their self-assembly. For instance, the case of equilibrium self-assembly of negatively charged single-stranded (ss) RNA with positively charged capsid proteins has been described based on fundamental physics, which yield assembly equilibria in good agreement with the experimentally observed behaviour [55]. It may be noted that the positive charge of the core-directed tail of the capsid protein does not suffice to neutralize the negative RNA charge, so that even for a rather small capsid, the negative core charge may still be in the range of x·1000 e0. Accordingly, electrostatics is very important for the interactions of viruses with their surroundings, and this issue has been addressed both experimentally (measuring forces by AFM) and theoretically [56]. It should also be noted that the size of a coronavirus and helical nucleocapsids in general increases in lockstep with the length of RNA [57]. Capsids are very well defined structurally, where many viruses have an icosahedral symmetry and are built from a given number of identical subunits. It might be noted here that the icosahedron is the regular polyhedron with the highest volume-to-surface ratio, i.e., optimised for packaging and fast diffusion transport of this packaged payload. The precise shape of polyhedral capsid shells is determined by the proteins it is built from and can be classified elegantly by the Caspar-Klug construction [58]. A comprehensive collection of capsid structures determined from cryo-electron microscopy and x-ray crystallography can be found at the VIPER website [59].
Of course, capsid formation by protein self-assembly is a highly interesting phenomenon in itself. A recent study of capsid growth around an RNA genome followed the growth process by an optical technique and showed that the process proceeds by nucleation and subsequent monotonic growth, and in the case studied, 90 chemically identical coat-protein dimers were assembled into a capsid. Both nucleation and growth time decrease with growing protein concentration, but the shortening is much more pronounced for the nucleation time. In any case, the time for the complete assembly was around 5–10 min, with concentrations of the coat-protein dimer in the range of 1–5 μM [60]. Accordingly, virus formation is a relatively fast process of complex self-assembly.
Whether these mechanistic steps of the self-assembly processes apply to other, more complicated viruses remains to be shown. Several viruses, such as influenza and coronaviruses, exhibit a different helical capsid structure. In the case of the influenza virus, the nucleoprotein (NP) oligomerises to form a double-helical hairpin structure, which contains polymerase proteins at one end. The genomic RNA is coiled on the outside of the helical capsid like on a spool [61]. As explained earlier, similar structures, the RNPs are present inside coronaviruses, but their precise structure still needs to be elucidated further.
General aspects of the interaction of surfactants with viruses
The interaction of surfactants with viruses has been a topic of investigation for many years, mostly driven by the interest of inactivating viruses by dissolving their lipid envelopes or denaturing proteins essential for their functioning, as the two main mechanisms. With the ongoing COVID-19 pandemic caused by SARS-CoV-2, this topic has suddenly regained the highest interest, as it brought into our focus that disabling virus activity can be essential for human survival. In the following, we want to lay out the basic physical chemistry of surfactants and their interaction with viruses, thereby providing the basics for how to use surfactants to suppress virus activity. Based on the fundamental aspects reviewed, we later sketch out directions in which future research activities might be directed to have more specific and efficient use of surfactants in working against the effects of viruses on human and animal health.
Thermodynamics
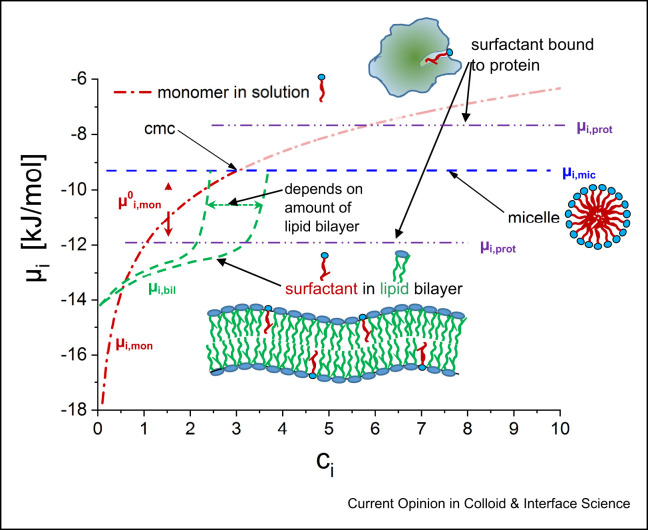
As discussed before, enveloped viruses have a variety of positions, where surfactants (they can be subdivided into anionic, cationic, and nonionic surfactants) can bind and interact, in particular the lipid membrane and membrane/envelope proteins. Looking at that aspect from a thermodynamic point of view, the likelihood and effectiveness of binding will depend in general on the affinity of the surfactant for the different binding sites at the virus (see Figure 2 ). In thermodynamics, this affinity is described by the chemical potential μi of a given species i (here, typically, the surfactant molecule is relevant). In thermodynamic equilibrium, μi of the surfactant molecules bound at various places to the virus has to be equal or lower for all positions of binding compared to that of the monomeric surfactant in solution, whose concentration dependence is described by:
| (1) |
Figure 2.
Chemical potential μi as a function of surfactant concentration ci. The red dash-dotted line gives the chemical potential of molecularly dissolved surfactant, according to μi,mon = μ0i,mon + RTlnai (μ0i,mon becomes more positive with increasing hydrophobicity of the surfactant, thereby increasing its tendency for binding to sites and lowering the cmc). Also shown are the levels for the chemical potentials of micelles (μi,mic), within a lipid bilayer (μi,bil) and two different potential binding sites on a virus protein (μi,prot).
(see Figure 2, ai: activity of species i, for the ideal case being identical to the mole fraction; R: ideal gas constant, T: temperature, μ0 i,mon: the standard chemical potential of the monomeric species, typically taken for the case of infinite dilution; its value will depend on the molecular architecture of the surfactant, being more negative for more hydrophobic surfactants, being more positive for more hydrophilic surfactants; accordingly the whole chemical potential curve can be shifted in y-axis by choice of the surfactant). Accordingly, the reference point that determines whether binding of surfactant takes place or not is naturally set by the concentration of free surfactant in solution. This means that for a surfactant to be effectively interacting with a virus, the chemical potentials of its relevant binding sites have to be sufficiently low so that this binding occurs at the lowest possible concentrations (see Figure 2, lower plateau for binding to protein). In particular, it should be below that of the micelles that are formed above the cmc (critical micelle concentration), as otherwise, any further addition of surfactant would become micellised but not bind directly to the virus (of course, micelles may also interact with viruses, see below). In contrast, the lower the chemical potential of the surfactant at the binding site, the lower the concentration at which the surfactant will be bound there — to potentially inactivate the virus. This means that ideally, the affinity of the surfactant to the virus binding sites has to be more pronounced than its tendency for micelle formation (μi,prot < μi,mic).
For enveloped viruses, a natural point of attraction for surfactants is the lipid bilayer into which surfactant molecules may become incorporated (defined in Figure 2 by μi,bil). The driving force will largely be hydrophobic interaction, but potentially also electrostatics may play a role, especially if the surfactant is oppositely charged (cationic). One may expect that at least initially, some surfactant may be bound within the lipid bilayer (see Figure 2), but its incorporation will raise the chemical potential of the surfactant in the bilayer, and one may expect that it becomes thermodynamically unstable once a certain percentage of surfactant is contained in the lipid bilayer. Controlling the extent of the partitioning of a surfactant into a lipid bilayer is its chemical potential μi,bil within that bilayer. This can be studied by calorimetric investigations, for instance, by isothermal titration calorimetry (ITC), where for SDS, an exothermic partition enthalpy of −25 kJ/mol (at 28 °C) for 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) bilayers has been reported, and this investigation also yielded an SDS-membrane equilibrium constant of 1.2–6·104 l/mol for the range of conditions investigated [62]. In contrast, nonionic and zwitterionic surfactants and free fatty acids have been reported to have endothermic partition enthalpies at room temperature [63].
For any binding site, hydrophobic and electrostatic interactions should play a role. In addition, especially for the interaction with biomolecules, more specific interactions may also play an important role, including short-ranged H-bonding (however, it should be noted that under physiological conditions also electrostatic interactions are rather short range, controlled by a Debye screening length of ~0.7 nm), whose strength depends strongly on the match of the molecular architectures, π-interactions, and potentially even forces due to complexation mediated by metal ions. A particular binding site would be the proteins in the outside structure of the virus, but given the varied structure of potentially relevant proteins, like M, N, E or S proteins (see 3.1), the binding affinity will depend largely on their detailed structure and state of assembly. Accordingly, the chemical potential μi,prot of the bound surfactant molecule can vary substantially from the micellised state (μi,mic) of the surfactant (Figure 2).
In biochemistry, the binding affinity is typically analysed via the Scatchard plot/equation that yields the dissociation or formation (binding) constant (both being the inverse of each other), the number of binding sites, and potentially information regarding the cooperativity of the process. For a process of two components, A and S (surfactant), forming a complex AS the formation constant K is given as:
| (2) |
with μ0 A, μ0 S and μ0 AS, being the standard chemical potentials of component (binding site) A, surfactant, and formed complex AS, respectively. It becomes clear from this formula that enhancing the standard chemical potential of the surfactant, μ0 S, for instance, by increasing its hydrophobicity by lengthening its alkyl chain, will, in turn, enhance the binding strength at a given binding site.
As an alternative way of interaction, above the cmc, surfactants can solubilise lipids or other molecules essential for the function of the virus within micellar aggregates, i.e., they can induce the leaching out of lipids from the membrane. This is to be expected above the cmc and would likely lead to inactivation of enveloped viruses, but typically also requires relatively high surfactant concentrations, which often one wants to avoid due to general surfactant toxicity.
Accordingly, in order to have surfactants that are efficiently inactivating viruses, either their chemical potential at the binding sites should be as low as possible, which will depend on the specific molecular interactions with either lipid membrane or protein, or one works with surfactants having as low a cmc/critical aggregate concentration (cac) as possible, This can be achieved by rendering the surfactant more hydrophobic and thereby increasing μ0 i,mon, which, from thermodynamics, is the key parameter to control surfactant activity. The latter is usually directly related to the length of the hydrophobic chain(s) and lower for nonionic than ionic surfactants [64]; however, for straight chains, one typically faces the problem of insolubility. Accordingly, the solution to achieve a low cmc would be employing branched chains or long chains containing double bonds. Viable alternatives are double-chain surfactants, such as typical phospholipids or dialkyl(ester) quats employed in fabric softeners [65]. They typically do not form micelles (due to their packing preference; having a cylindrical shape), but vesicles are often present above their critical aggregate concentration, and the cac is normally in the nM to μM range. A further alternative are amphiphilic copolymers of block or graft structure, which generally have very low cmc values [66]. Such polymer surfactants often interact strongly with lipid bilayers, and even such highly biocompatible copolymers of the PEO-PPO-PEO (Pluronic ©) type have been shown to disturb lipid bilayers substantially [67].
For maximizing the binding affinity of a surfactant to virus components, one has to mainly consider that lipid membranes, due to the presence of sialic acid-containing glycolipids, are generally negatively charged and that cationic surfactants are most strongly attracted, which also explains the generally high biotoxicity of cationic surfactants compared to others. As a basic rule, the affinity of a surfactant to become incorporated into a lipid bilayer (quantified by μi,bil) will depend largely on the molecular architecture of the surfactant and on the lipid composition of the bilayer. It can be described by a distribution coefficient that gives the probability for being in a bilayer compared to being in the corresponding aqueous solution. Such distribution coefficients can be measured experimentally, but their determination is not necessarily simple and becomes more challenging if the affinity is very high. The most direct and reliable way to determine the thermodynamics of the surfactant/bilayer interactions is certainly isothermal titration calorimetry (ITC) that gives not only the partition coefficient, but also enthalpy, entropy, and free energy of the transfer process of the surfactant into the bilayer [68]. More recently, however, the theoretical calculation of distribution coefficients based on molecular simulations has also been advanced, for instance, for describing the sorption data of single-chain and double-chain cationic surfactants. This approach also allows handling of more strongly membrane attracted cationic surfactants [69].
By comparison, the binding to proteins is much more complex. The binding at different protein sites will have very different effects with respect to inactivation. Binding to some sites may have no effect at all, while binding at others may effectively disable protein function altogether. This means that smart molecular design requires determination where surfactant should bind at a given protein. For the binding, electrostatic and hydrophobic interactions may be most relevant but H-bonding can also be important, as also even more specific molecular interactions. This will largely depend on the molecular details of the protein binding site and, hence, structural features with atomistic resolution will be important as they can be obtained from highly resolved scattering/diffraction experiments and NMR, but also from molecular modelling approaches.
Investigations on the thermodynamics of the interaction of surfactants with viruses are rare. In one notable exception, human influenza (H3N2) and avian influenza virus strains (H5N3) were investigated with respect to the effect of the surfactants potassium oleate (KOl), SDS, and sodium Laureth sulphate (SLES). Interestingly, infectivity was reduced most dramatically by KOl, much less by SDS, and still somewhat less by SLES. A parallel investigation by isothermal titration calorimetry (ITC) for KOl showed a marked exothermic interaction with the H3N2 virus, while it was less exothermic for SDS, and even endothermic for SLES. This was interpreted such that the interaction is dominated by electrostatics in the case of KOl, while for SLES, hydrophobic interactions prevail [70]. This investigation nicely shows that fundamental thermodynamic investigations can help in understanding the interactions relevant to reducing the infectivity of viruses.
Kinetics
While we discussed the thermodynamics of an equilibrated situation for virus inactivation by surfactants in the previous chapter, the other essential issue is the time scale at which the interaction/binding will take place. This means we must look at the kinetics of surfactant–virus interaction, which determines how fast such an equilibration is achieved.
The main point here is that a surfactant molecule must diffuse to a given binding site of a virus. That process has basically been described by von Smoluchowski more than 100 years ago [71]. Typically, diffusion-controlled reactions are very fast, but for some situations in surfactant–virus interaction, this may no longer be the case as the virus concentration is necessarily low. If surfactant concentration is equally low, and for many applications, one may target a low cmc, for example, for reasons of surfactant toxicity or ecological concerns, the reaction times may become very long. The mean time τ required for one virus particle of radius RV to be hit by a surfactant molecule can be estimated as:
| (3) |
where cS is the surfactant concentration, Ds the diffusion coefficient of the surfactant, and NAv Avogadro’s constant. Assuming a surfactant concentration of 0.1 mM, DS of 2∗10−10 m2/s (neglecting the movement of the virus as its diffusion coefficient is more than 50 times less than that of the surfactant), and a virus radius RV of 50 nm, a characteristic time of ~130 ns can be calculated, which means a surfactant molecule encounters a virus particle every 130 ns. It might be noted here that, naturally, the time required for micelles to encounter a virus will be much longer, by a factor larger than their aggregation number, simply due to the correspondingly lower concentration of micelles. If we now look more quantitatively at the interaction of the surfactant with the lipid envelope, we have to consider that for a radius of 50 nm, the virus envelope will contain about 125,000 lipid molecules under the assumption that their head group area is approximately 0.5 nm2. This, in turn, means that for substituting just 1% of the lipid molecules with surfactant molecules, it would take about 0.15 ms. This is assuming the best-case scenario that every encounter leads to incorporation of surfactant into the bilayer, while, in reality, one may expect here a certain activation energy to be required that would slow down this process substantially.
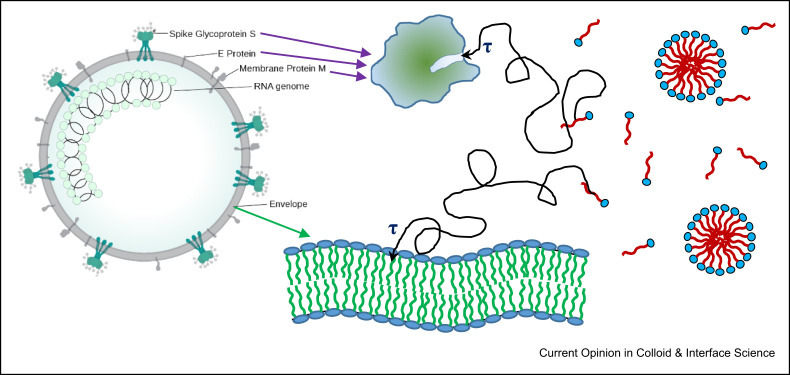
Of course, the time required for a surfactant molecule to encounter a protein by diffusion will be much longer as the protein is much smaller. One may estimate it from eq. (1) by assuming a protein size, where a radius of 2–5 nm is realistic, but in addition, the proteins might also be partly protected by being incorporated within a bilayer or a larger protein assembly, so only a part of it is free for contact. Somewhat different is the situation for membrane proteins, as the mobility of surfactants within a bilayer is very high, and accordingly, the ease of encountering a membrane protein bind in the membrane. In any case, just being in contact is not enough for a surfactant to bind and interact further, because first, the probability of hitting the virus surface at the proper place for binding (e.g., location of a protein, see Figure 1) is not necessarily very high; it is certainly much higher for hitting the lipid membrane than for a specific protein. Second, making contact does not mean binding, as there will always be steric effects. For instance, binding of a surfactant molecule to a protein requires the proper orientation at the right position of the protein and an activation energy Ea must be exceeded before binding can take place. Both aspects can be accounted for by an effective activation energy Ea,eff that is reducing the rate of this binding process by a factor exp(−Ea,eff/kT), compared to the probability of just having a collision, which would be the maximum possible rate. This concept applies similarly to the incorporation of surfactant into the lipid bilayer, but this process will be much faster due to the fact that by area, the lipid envelope is much bigger and Ea,eff should be much lower as the incorporation of a surfactant molecule into an existing bilayer is relatively easy.
Surfactant interaction with model lipid bilayers
Liposomes are vesicles composed of a lipid bilayer, which are often used as model systems for biological membranes. Their shell consists of lipids only and is missing the proteins, cholesterol and other building blocks of real membranes, which makes them suitable for simplified experimental membrane studies. A lot of research has been carried out on the interactions between surfactants and phospholipid bilayers that ranges from coexistence to incorporation of the surfactant into the lipid bilayer to solubilisation of the membrane into mixed aggregates.
A general description of the processes occurring with increasing surfactant to membrane ratios was proposed by Helenius and Simons as early as 1975 [72] as the ‘three-stage model’: In stage I, at low surfactant concentrations, the surfactant is incorporated into the bilayer and causes changes in its physical properties. Such changes may include different spontaneous curvatures as the typical surfactant has a relatively large head group — inverted cone shape, imperfections in the highly ordered lipid arrangement, increased permeability and even ruptures of the bilayer. This is found already way below the cmc of the respective surfactant as there typically is an affinity of the surfactant to become incorporated into such a bilayer (see Figure 2, Figure 3 ). Upon increasing surfactant concentration, more surfactant is incorporated in the bilayer, while the bulk concentration of surfactant stays below the cmc, and no micelles are formed. In stage II, when the bilayers are saturated with surfactant, the bilayers begin to break, and mixed surfactant/phospholipid aggregates are formed, mostly in the form of large cylindrical micelles. The mixed cylindrical micelles coexist with the remaining membranes at first, but with increasing surfactant concentration, this eventually results in a complete phase transition. In stage III, after completed phase transition, the surfactant/phospholipid ratio in the mixed micelles increases while their size decreases. This is often realised by a transition from cylindrical to small spherical micelles.
Figure 3.
Different (simplified) ways of surfactant interacting with different virus components. Depicted are diffusion paths with a characteristic time τ (as discussed above and described by eq. (2), (3) strongly depending on surfactant concentration and typically in the range of 50 ns–1 μs) required for the surfactant to migrate its way to the binding site at bilayer or protein, respectively.
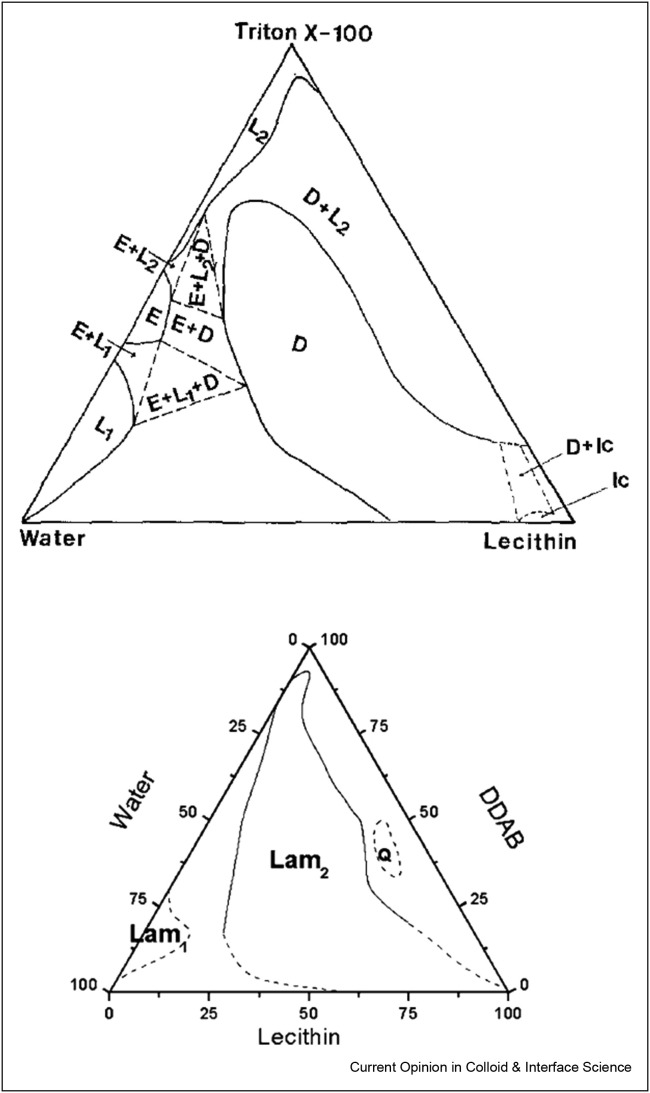
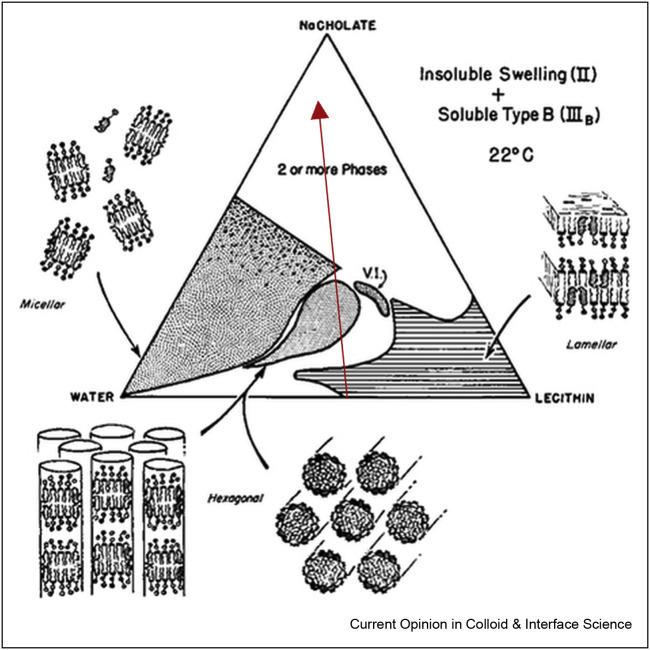
The solubilisation of membranes by surfactants was studied, for example, for the model system of lecithin/DDAB/water [73]. Lecithin is a zwitterionic lipid, which is practically insoluble in water but forms lamellar liquid crystalline structures at high concentrations. These lamellar structures can serve as a model for biological membranes. DDAB (dialkyldimethylammonium bromide) is a cationic surfactant with two carbon tails. The ternary phase diagram of this system is shown in Figure 4 a. Here, the three corners represent the pure components (surfactant: top, water: bottom left, lecithin: bottom right), the borders of the triangle show two-component mixtures in wt%, while the inside area corresponds to all ternary compositions possible. The phase diagram shows a large area with lamellar structures (Lam2), which is not easily dissolved by DDAB. Only at very high DDAB concentrations, a two-phase region is formed, and a homogeneous micellar L2 phase is never reached. The phase behaviour was also studied for the cationic surfactant DTAB, which is similar to DDAB but has only one carbon chain [74]. With DATB, it was possible to dissolve the lamellar lecithin structures into mixed micelles. This effect can be attributed to the smaller packing parameter of DTAB compared to DDAB, which is more effective in disrupting the lecithin arrangement.
Figure 4.
Ternary phase diagrams of lecithin/DDAB/water and lecithin/Triton X-100/water. The lamellar phases are labelled as Lam2 and D, respectively, and micellar phases are called L1 and L2. Reprinted with permission from Ref. [73] Copyright 2002 American Chemical Society and from Ref. [75], Copyright 1989, with permission from Elsevier.
The phase diagram of a similar system with the nonionic surfactant Triton X-100 is shown in Figure 4b and looks slightly different [75]. It shows a large area with lamellar structures (D), that, at higher Triton X-100 concentrations, turns into a two-phase region of lamellar structures coexisting with micelles until finally, at very high Triton X-100 concentrations, a mixed homogeneous L2 phase is formed. These findings follow nicely the three-stage model described by Helenius and Simons, but it can be seen that very high surfactant concentrations are needed here to dissolve the lipid bilayer.
In order to achieve lipid bilayer disruption, single-chain surfactants are more effective than double-chain surfactants and nonionic surfactants have a bigger solubilization power than cationic. But the best results in breaking phospholipid membranes are achieved by anionic surfactants, as shown for the system of lecithin/sodium cholate/water in Figure 5 . Bile salts are popular surfactants for lipid membrane solubilisation studies since they are natural, biological compounds and highly effective in solubilising lecithin and other lipids. Only one mol of sodium cholate can solubilise two mols of lecithin [76], whereas, for example, two [77] or 1.5 mol [78] of Triton X-100 were claimed to be required for solubilisation of 1 mol lecithin, irrespective of the total concentration of lipid. Figure 5 shows the ternary phase diagram of lecithin/sodium cholate/water mixtures. It shows that lecithin in water forms lamellar structures, a model for biological membranes. Upon addition of the anionic surfactant, Na-cholate in this case, the lamellar membranes are dissolved, and mixed cylindrical aggregates are formed (red arrow).
Figure 5.
Ternary phase diagram of lecithin/sodium cholate/water and corresponding structures. The red arrow shows the dissolving of lipid bilayers upon the addition of surfactant. Reprinted with permission from Ref. [76] Copyright 1968 WILEY.
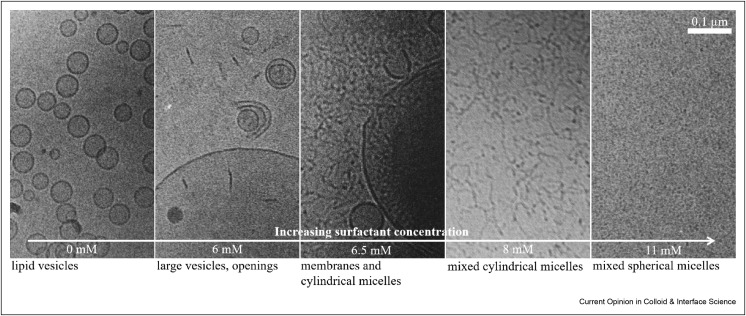
While these phase diagrams give a good overview of all the possible structures occurring in a three-component lipid/surfactant/water system, they give little information on the changes on the bilayer at surfactant/phospholipid ratios lower than needed to obtain complete phase transition from the lamellar to the isotropic micellar phase. Walter et al. [79] have studied the vesicular/micellar transition of cholate-egg PC vesicles with increasing cholate content, using cryo-TEM. Images taken at different cholate concentrations nicely show the different stages of bilayer solubilisation described by Helenius and Simons (see Figure 6 ). After the addition of low levels of Na-cholate, the vesicle structure was maintained, but their morphology was altered. At just slightly higher cholate concentrations, openings of the bilayer sheets were observed. Long flexible cylindrical structures coexist with open vesicles over a wide range of concentrations before, finally, at high cholate concentrations, no bilayers were present anymore, and the cylindrical structures transformed into small spherical micelles of ~4 nm diameter. A similar structural evolution, documented by a combination of static and dynamic light scattering with cryo-TEM, has also been reported for the addition of dodecyloctaglycol (C12E8) to egg PC. Here, the formation of worm-like micelles sets in above a concentration of 40 mol% C12E8 [80]. One might expect the formation of disk-like micelles as intermediates, but interestingly comprehensive work has shown that typically worm-like structures are formed once the lipid vesicles become dissolved [81]. Lipid solubilisation by the micellar mechanism typically proceeds such that the surfactant does not migrate from the outer phospholipid leaflet of a vesicle to the inner one. The excess of surfactant molecules in the outer monolayer then leads to shape change and disruption of the bilayer structure [82,83].
Figure 6.
Cryo-TEM images of egg PC vesicles (c = 9 mM PC in all images) with increasing surfactant concentration (sodium cholate)from left to right. Scale bar is valid for all images. Reproduced from Ref. [79] Copyright 1991, with permission from Elsevier. Inspired by Ref. [84].
The most common effect of added surfactant on membranes is a curvature stress becausemost surfactants have a rather large head group and one hydrophobic tail (inverted cone shape, see 2.3), and therefore, possess a packing parameter, favouring the formation of (spherical) micelles. Accordingly, their incorporation disrupts bilayer formation, but the effect typically requires the presence of 30–60 mol% of surfactant in the lipid bilayer. Studies using fluorescence probes and determining limiting fluorescence anisotropy, which is related to the membrane order, have shown that for typical synthetic surfactants, a continuous decrease of membrane order is observed with increasing surfactant content, a process referred to as homogeneous disordering. In contrast, for several antimicrobial biosurfactants such as lipopeptides and saponins, membrane lysis is observed without such a prior decrease of membrane order. It has been speculated that the lipid membrane becomes disrupted locally by surfactant-rich defect structures [85]. This different mechanism then would also be an explanation for their effectiveness in membrane lysis already at much lower concentrations. Of course, this different mechanism of interaction has nothing to do with the biological origin of these surfactants, but their more complex structure disfavours homogeneous mixing with the lipid in the membrane and instead favours segregation within such a membrane.
Theoretical work on the interaction of lipopeptides with different types of lipid membranes simulating bacterial versus mammalian membranes showed that the overall binding affinity is largely determined by the length of the hydrophobic part, while the peptide part regulates the selectivity of binding to the different types of membranes [86]. The time scales involved in detergent solubilisation of lipid bilayers can vary significantly, and this has been attributed to the ability of the surfactant to perform a trans-bilayer movement (flip-flop), and only ones that can initiate this process rapidly will result in fast bilayer solubilisation [87]. Model experiments by means of time-resolved stopped-flow SAXS experiments on small, extruded lipid vesicles showed that the effect of adding an excess amount of sodium dodecyl sulphate (SDS) leads to the dissolution of the bilayers, which can be described by two characteristic times, one for complete adsorption of the SDS on the liposome surface, and one for the desorption of the first mixed micelles. These times depend on the charge state of the bilayer and are faster for positively and slower for negatively charged vesicles. However, in general, vesicle dissolution takes place within 10–30 s [88].
Interactions of surfactants with proteins
Besides lipids, proteins are the other major building blocks of biological membranes. In general, surfactants are well known to bind to proteins, the main driving forces being electrostatic, hydrophobic, and H-bonding. Given the large number of structurally different proteins and surfactants, the topic of surfactant–protein interactions is a very large and fast-evolving field, which cannot be covered in further depth here, but was reviewed some time ago [89]. The binding of a surfactant often leads to denaturation of the protein, either by unfolding or by the formation of protein-surfactant complexes [90]. Here the strength of the binding will depend on the molecular architecture of the protein binding site and how well the surfactant is adapted for that. In contrast, in the case of membrane proteins, the presence of lipids/surfactants is essential for proper functioning. Accordingly, virus functions that rely on the activity of membrane proteins can only be expected to be affected by surfactant interactions with the lipid virus envelope into which such membrane proteins are embedded. However, the effects on membrane properties can be expected to be weak as long as no larger percentage of surfactants is incorporated. An alternative would be extracting membrane proteins selectively from the bilayer, where the detailed surfactant structure will be important to achieve the highest possible affinity of the surfactant for membrane proteins. On the other hand, capsid proteins are also essential for virion functionality and vulnerable to interactions with surfactants. However, they are protected in the case of enveloped viruses, where any added surfactant will first have to encounter and interact with the lipid envelope before it would be able to reach and interact with capsid proteins. And as discussed before (see 2.4), the crossing of a membrane, i.e., the jump from the outer to inner monolayer (flip-flop), is typically a very slow process.
Rather well studied has been the binding of fatty acids to proteins, as there is also a particular class of fatty acid-binding proteins (FABP), which are involved in fatty acid transport and metabolism. Interestingly fatty acid-binding is largely driven by enthalpy, where van’t Hoff enthalpies of ~−40 to 50 kJ/mol were observed for fatty acids such as oleic acid, arachidonic acid, or palmitic acid, the precise value depending to a certain extent on the origin of the FABP [91]. This is different to the typical hydrophobic association, which is driven by entropy [92]. The binding affinity and the corresponding Gibbs free energy of binding are systematically increasing with increasing hydrophobicity of the fatty acid. This has similarly been observed for the binding of different fatty acids to bovine serum albumin (BSA), for which also binding enthalpies of −50 to −30 kJ/mol were reported, but the process becoming less exothermic with increasing length (hydrophobicity) of the fatty acid [93]. However, as discussed in Chapter 4, not only the thermodynamics of this binding process but also the kinetics are relevant. Here for different fatty acids, a kinetic of off-rate constant in the range of 0.5–5 s−1 has been observed at room temperature, actually becoming faster for fatty acids of increasing chain length [94], which is a bit counterintuitive. However, in any case, these complexes are rather long-lived.
Surfactant interactions with virus membranes
The main component of virus envelopes are lipids, and like all biological membranes, they can contain a large variety of different lipids, with their main component being phospholipids. However, the detailed lipid composition of viral envelopes can vary largely, as has been shown for the case of different strains of influenza virus, which vary largely with respect to the types of glycerophospholipids contained (head groups) and the amounts of saturated and unsaturated acyl chains contained in the respective lipid type [95].
However, even if biological membranes are extremely complex mixtures of many lipids, cholesterol, proteins, ions, etc., the general concepts of surfactant/membrane interactions described above for simple phospholipid bilayers also apply to virus membranes. In addition, specific surfactant/protein interactions may occur, as well as preferred solubilisation of different membrane components. For instance, it has been observed for lipid bilayers containing Ca2+-ATPase that SDS extracts the Ca2+-ATPase from the membrane before actually solubilising the lipids. In contrast, nonionic surfactants like C12E8 or Triton X-100 predominantly interact with the lipids [96]. This demonstrates that the choice of the surfactant is of central importance for the observed interactions. Here, it is mainly a charge effect, but the length of the hydrophobic part and other molecular details of the whole surfactant molecule will also be of importance. A lot of research on surfactant/membrane interactions was carried out with the goal to solubilise and isolate lipids or proteins from the membrane. But this knowledge can be transferred to virus inactivation, as alterations in the lipid envelope will certainly affect virus functionality and with it infectivity.
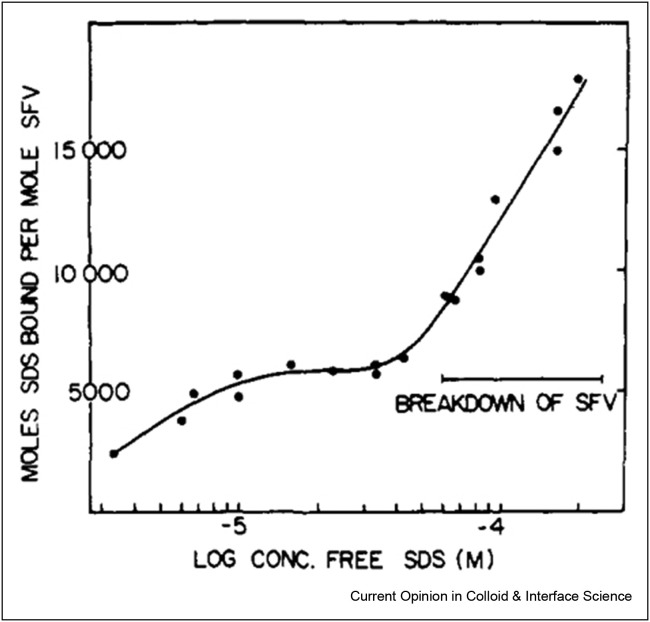
A nice example is the solubilisation of the Semliki Forest Virus (SFV) membrane upon addition of the anionic surfactant SDS, which was studied by Becker et al. by density gradient centrifugation [97]. Lipids floated on the top while DNA sedimented to the bottom of the gradient as identified by radioactive labelling. From the fractions of lipids and DNA collected, the different stages of virus breakdown could be reconstructed (Figure 7). When SDS is added to SFV in low concentrations, it starts binding to virus particles but without any signs of disruption. Virus disruption started after binding about 6000 SDS molecules. Assuming the virus membrane consists of only 16,000 phospholipids and 16,000 cholesterol molecules [98], the disruption would start at just under 20% substitution of membrane molecules. Higher SDS concentrations led to complete virus breakdown when the viral nucleocapsid dissociated into RNA and protein, but the exact SDS concentration needed for virus inactivation could not be determined. A comparison with the nonionic surfactant Triton X-100 shows the milder impact of nonionic compared with charged surfactants on membranes [99], which may be attributed to the fact that nonionic surfactants have only a small effect on the charge conditions of the membrane, which are critical for overall membrane organisation and proper functioning.
Figure 7.
Binding isotherm of sodium dodecylsulphate (SDS) to Semliki Forest Virus (SFV) at 4° determined by sucrose gradient centrifugation. Reprinted with permission from [97]. Copyright 1975 American Chemical Society.
Regarding coronaviruses, especially the S glycoproteins might be a weak point as S damage will lead to a loss of virus infectivity, as was demonstrated for the murine hepatitis coronavirus MHV-A59 [100]. It has been reported for SARS-CoV-2 that the receptor-binding domains of S tightly bind the linoleic acid in three composite binding pockets. This binding apparently reduces virus activity as one observes a synergistic effect and enhanced suppression of SARS-CoV-2 replication when treatment with the polymerase inhibitor remdesivir is combined with linoleic acid addition [24].
In addition, several glycoproteins of enveloped viruses interact with cholesterol within the membrane, and this interaction is vital for the function of the protein during virus entry by membrane fusion and virus release by budding [20, 21, 22]. Replacing the natural cholesterol in the virus membrane with other sterols has been shown to affect the rate of membrane fusion and hence might inhibit virus entry [101].
Existing investigations and applications of surfactant use against viruses
Quantification of (infectious) virus particles
The central point in assessing the effectiveness of a surfactant against a given virus is the quantification of viral activity after such a treatment. For investigating whether a particular procedure affects the infectivity of a virus preparation, the number of infectious virus particles must be quantified. A classical virological approach is the plaque assay. A plaque develops when adjacent cells are killed by virus replication, starting with a single infectious particle. For quantifying the number of infectious virus particles, the virus-containing solution is serially diluted, and then the separate cell monolayers are inoculated with the dilutions. After virus entry (approximately 1 h), the monolayer is overlaid with a semi-solid medium (e.g. agar) to allow the virus to spread only to adjacent cells. After incubation of the cells for 2–3 viral replication cycles (2–7 days, depending on the virus), cell monolayers are treated with a vital dye that stains only live, uninfected cells. If the number of infectious viruses in the inoculum is equivalent to or exceeds the number of cells in the monolayer, every cell will become infected and killed, and hence, the whole monolayer remains colourless. At a certain virus dilution, only a few particles remain, each of which infects a single cell and its progeny are spread to neighbouring cells. Individual plaques are visible to the eye as a colourless spot against the background of a stained monolayer. The number of plaques is multiplied by the reciprocal value of the virus dilution to calculate the number of infectious virus particles. A variation of the plaque assay is the tissue-culture infectious dose 50 (TCID50) assay, where the cell monolayer is not covered by an overlay, and hence eventually, all cells become infected even if only a single infectious particle is present in the inoculum. Nevertheless, infection titers determined with TCID50 tend to be somewhat lower than those determined with a plaque assay. Since both assays only count virus particles capable of undergoing a complete replication cycle (virus entry, viral genome replication, viral protein synthesis, and production of infectious progeny particles), they are the gold standard for determining virus infectivity. However, virus titers depend on the cell type used for the plaque assay, i.e., results obtained with the same solution titrated in different cell types may differ by as much as 10-fold. Note that only a small fraction of physical virus particles visible in the electron microscope (10%–0.01%) are infectious. Furthermore, the infectivity of most enveloped viruses is very labile; an influenza virus preparation shows a titer reduction of ~1 log (=90%) when incubated at room temperature for two days.
Initial steps of virus replication (virus binding and entry) can also be studied using fluorescence-based methods, such as fluorescence-assisted cell sorting (FACS) and fluorescence microscopy. They are more sensitive, quantifiable and can be scaled up and automated but require specialized equipment and expertise.
In summary, there is no definitive answer to the seemingly simple question about the number of infectious particles in a virus preparation. It depends on the assay and cell types used. Methods that quantify viral genome copy number, such as PCR, are not suitable for determining the infectivity of viral particles because neither lipid- nor protein-targeted treatments affect the viral genome, and hence, the genome copy number remains the same before and after treatment.
Disinfection of hard surfaces and skin
Coronaviruses seem to be remarkably stable on various surfaces. Several studies for SARS, MERS and other human coronaviruses show that the viruses can remain infective for up to 9 days on many common hard surfaces, such as steel, glass or plastic, although virus titres dropped significantly during that time [102]. Therefore, disinfection of frequently touched surfaces is key to stopping virus spread. While common disinfectants mainly contain ethanol or isopropanol, it was shown that the addition of surfactants increases virucidal efficiency [103]. The different types of sanitising agents for virus inactivation and disinfection have been reviewed recently, including which types of surfactants are used but also with a large range of other chemical compounds employed in that context [104]. A long while ago also specifically, the inactivation of Coronaviruses has been reviewed [105].
Due to its importance, the field of disinfecting virus-contaminated surfaces is one that has been investigated thoroughly for a long time, and surfactants traditionally play an important role when it comes to disinfection [106]. A recent review not only describes in a comprehensive fashion the antiviral activity of surfactants but also other health-related functions of surfactants, like antimicrobial activity or anti-oxidative properties [107], and we have summarised the main existing or developing antiviral applications of surfactants in Table 1 .
Table 1.
Different surfactants with antiviral activity and observed effects, classified according to their type (anionic: a, nonionic: n, cationic: c, zwitterionic: z).
| Name | Type | Effect | Ref. |
|---|---|---|---|
| soap (Na oleate, laurate) | a | Inactivation of Flu viruses | [109] |
| SDS | a | Reducing the infectivity of tomato bushy stunt and potato X viruses | [110] |
| Disinfection | [106,107] | ||
| Triton X-100 | n | Activity of the replication/transcription complex of SARS-CoV was greatly reduced | [115] |
| Effectiveness of classical, often surfactant-based, disinfectants | [116] | ||
| Sodium Laureth sulphate | a | Inactivate SARS-CoV-2 effectively | [117] |
| Quaternary alkyl ammonium salts (QUATs) | c | Disinfection; most widely employed group of disinfectants for hard surfaces | [120, 121, 122, 123] |
| Hand sanitation | [128] | ||
| Benzalkonium chloride | Inactivates Coronaviruses | [118,119] | |
| Chlorhexidine (typically as salt, e.g. as gluconate or digluconate) | c | Antiviral activity against enveloped viruses, as demonstrated for SARS-CoV-2 | [138] |
| Chlorhexidine together with CPyC | c | Effective against SARS-CoV-2 in a variety of different formulations | [139] |
| Different agents in mouthwashes/dentistry | [140] | ||
| Anionic peptide surfactant surfactin | a | Very effective against enveloped viruses but: applicability is limited by cytotoxicity |
[134,135] |
| Sophorolipid | a/n | Antiviral activity, e.g., against HIV | [136,141] |
| Rhamnolipids | a | Antiviral activity, e. g., against HSV | [142] |
| Antiviral activity and additive to mask fabrics | [143] | ||
| Saponins | c | Antiviral activity against many viruses, including coronavirus H-CoV-22E9 | [137] |
| Biosurfactants | [144] | ||
| Nonionic surfactants like Tween-80, Span-20 | n | Nanoemulsions or microemulsions effective as antiviral formulations | [145] |
| Nanoemulsion surfactant mixture of Triton X-100, TBP, CPyC | a, n, c | Inactivation of Ebola virus | [120] |
| CPyC | c | Likely candidate for effectively disabling enveloped viruses, including SARS-CoV-2 | [122,123] |
| Lipids, phospholipids | z | Delivery vehicles of many vaccines | [146, 147, 148] |
| Artificial pulmonary surfactant | Inhibiting alveolar collapse and diffuse alveolar damage in the lungs | [149] | |
| Phosphatidylglycerol plus lung surfactant | To restoring lung surfactant damaged by infection | [150] |
In general, the role of surfactants is not just that of interacting with the lipid envelope (and typically dissolving it) or of denaturing proteins. In most practical situations on a dried surface, one may not encounter individual viruses but mainly agglomerates, in which many viruses are lumped together. The function of surfactant then is to dissolve such agglomerates and render the individual viruses accessible, which means that surfactant activity is quite similar to that in one of its principal actions in detergency. Otherwise, the viruses inside of such agglomerates would be rather well protected from any type of inactivation [106,108]. Accordingly, surfactants can also be very important as an ‘access facilitator’ in formulations with other antiviral components by allowing them to access individual viruses more easily.
Absolutely classic in the context of disinfection is the use of soap for inactivation of influenza viruses shown to be effective around neutral pH more than 80 years ago [109], i.e., before even knowing much about virus structure. In addition, conventional anionic detergents like SDS were also shown early on to be similarly effective in reducing the infectivity of tomato bushy stunt and potato X viruses [110]. The rather general activity of SDS against enveloped (for instance, HIV) and nonenveloped viruses, which has already been reviewed some 20 years ago, has been assumed to be due to denaturing the lipid envelope and/or the capsid proteins, and generally, its antiviral activity is already present in non-cytotoxic concentrations [111]. As anionic surfactants are primarily active against enveloped viruses but not against nonenveloped viruses, it can be concluded that they primarily interact with the lipid bilayer [106], despite the fact that the lipid bilayer itself also has an anionic character. In general, it has been observed that enveloped viruses are more sensitive to the interaction with chemical agents such as surfactants than nonenveloped viruses [112]. It might also be noted that liquid soaps, e.g. made from sodium lauryl ether sulphate and cocamidopropyl betaine, are more effective against nonenveloped viruses such as human noroviruses than ethanol-based hand sanitisers, while for the enveloped viruses, the opposite behaviour has been observed [113]. This can be explained such that alcohols like ethanol or isopropanol are very effective in dissolving lipid bilayers but have only very little effect on nonenveloped viruses, whereas surfactants work on both types of viruses but are also more effective against enveloped viruses. In that context it should also be noticed that simply distinguishing the antiviral activity with respect to having an enveloped or nonenveloped virus is oversimplifying the situation as a different response is also seen for different enveloped viruses and also with respect to employing different surfactants, as for instance, demonstrated for cationic compounds [114].
When looking more specifically at coronaviruses, the activity of the replication/transcription complex of SARS-CoV was greatly reduced by the presence of the nonionic surfactant Triton X-100, indicating that its effect on lipid membranes rendered this complex inactive, thereby inhibiting virus replication [115]. Similarly, for SARS-CoV-2, the effectiveness of classical, often surfactant-based, disinfectants has been ascertained [116]. Even simple household surfactants like sodium Laureth sulphate (commonly found in shampoos) have been shown to inactivate SARS-CoV-2 effectively at low concentrations of 0.1 wt% within 60 s on a large variety of different inanimate surfaces [117].
In comparison, cationic surfactants are more established bactericidal compounds and quaternary alkyl ammonium (QUAT) compounds are commonly employed for disinfection, e.g. benzalkonium chloride [118,119]. The antiviral action or didecyldimethylammonium chloride, but also pyridinium, imidazolium or isoquinolinium surfactants, have been intensely studied in that context [120, 121, 122, 123].
QUATs are the most widely employed group of disinfectants for hard surfaces. However, even though they are very potent antimicrobial agents, they are generally much less effective in inactivating viruses [124]. This is particularly evident for nonenveloped viruses but still markedly observed for enveloped ones, especially after they have been dried on surfaces. However, antiviral activity can become enhanced by rendering the systems more acidic by addition of HCl [125]. This aspect has been critically discussed [126] and comprehensively reviewed recently [102]. The main reason for the lower effectivity of QUATs may be attributed to the fact that their main effect in antimicrobial activity is to modify cell permeability [102], an effect much less relevant for inactivating viruses, as the virus lipid envelope is mostly for protection and transport across the bilayer is a property of lesser importance. The particularly low efficacy of cationic surfactants with respect to viruses under dry conditions on surfaces may also be attributed to the fact that here, in addition to the interaction with the virus lipid envelope, one has first to disperse the viruses, like dirt in detergency. However, both typical surfaces and viruses will be negatively charged, and therefore, cationic surfactants will perform rather poorly in dispersing them, similar to their relatively poor performance in typical detergency. Nonetheless, the use of QUATs for disinfection has naturally been increasing substantially during the recent pandemic and has been raising concerns regarding their environmental impact [127]. Certainly, a very important aspect for future developments is having formulations that are effective with respect to their antiviral properties but at the same time mild with respect to toxic effects, and do not come with the risk of environmental damage, as recently discussed in the case of hand sanitation [128].
The field of hand sanitation is, of course, a central one in controlling virus spread. Regular hand hygiene is very important [129], but typical hand sanitisers are rather harsh surfactants, as is the case for standard alkaline soaps. Accordingly, their frequent application will lead to compromised skin by removing lipids from the stratum corneum, and such damaged skin then may become itself more penetrable to viruses and other damaging particles, and one also increases the risk of hand dermatitis. Therefore, working at pH 5.5 to 7.0, i.e., that of healthy skin and use of mild surfactants, is recommended [130].
On the side of studying the effectivity of surfactants against virus activity, recent studies have been dedicated to not just using single surfactants but combinations thereof in order to benefit from synergistic interactions, similar as is conventionally done in detergency, where typically anionic surfactants are combined with a nonionic surfactant to reduce the cmc, while retaining the negative charge required in detergency. This was successfully shown for mixtures of the cationic didecyldimethylammonium chloride (DDeCl) with dodecyloctaglycol (C12E8) and cyclodextrin (CD) [131]. Marked synergism in antiviral activity was reported, and a 4-log10 virus inactivation within 15 min contact time was seen for concentrations around the cmc.
As just discussed, many surfactant mixtures are quite effective with respect to their antiviral properties. Accordingly, there is a very large number of commercial products on the market, mostly for use in hospitals, industrial disinfection or preservation of food, which rely on surfactants and mixtures thereof. A rather comprehensive and regularly updated list can be found on the website of Health Canada [132].
However, it should be noted that the disinfection of large surface areas requires extensive use of surfactants, which will ultimately end up in the environment. It is, therefore, necessary to increase the use of sustainable and biodegradable surfactants in this area [133]. Promising compounds here are e.g. surfactin or rhamnolipids and sophorolipids, which have all shown antiviral activity against various enveloped and nonenveloped viruses [134, 135, 136]. Also, saponins, which are plant-derived biosurfactants, were shown to possess antiviral activity against many viruses, including coronavirus H-CoV-22E9 [137].
Antiviral use of surfactants in formulations for personal care etc.
While disinfection of hard surfaces is an important topic in circumstances where rooms, instruments, hands, etc. have to be sterile and safe, a related topic is the use of surfactants in formulations for personal care, which then become important in protecting humans. The main difference with respect to requirements for the surfactant is that for hard surfaces, one can accept rather harsh conditions; for personal care, it is important that the surfactant is as ‘mild’ as possible, where, of course, mild depends on the environment, where it is supposed to be applied, being different for skin versus in the mouth or nose.
A frequently employed disinfectant agent in that field is chlorhexidine (typically in the form of its salts, e.g. as gluconate or digluconate), which is not a proper cationic surfactant but forms aggregates in solution [151]. It is a regular component in many pharmaceutical products (e.g., antiseptic mouthwashes), cosmetics or disinfectants, and shows antiviral activity against enveloped viruses, as demonstrated for SARS-CoV-2, where complete inactivation within 30 s was observed, further enhanced by the presence of molecular iodine [138]. Here an interesting study by neutron diffraction, using a model membrane of PC, has shown that chlorhexidine positions itself at the head group of the phospholipid, suggesting a mode of action where the molecule is bent in half and inserts wedge-like into the lipid matrix, a mode of action that is very similar to that employed by antimicrobial peptides when they interact with bilayers [152]. Chlorhexidine is often employed in combination with CPyC, which has been known for a long time to be a very effective microbial for mouthwash formulation, but recently was also shown to be very effective against SARS-CoV-2 in different formulations [139]. The reduction in virus titer was very high (between approximately 1000- to 10,000-fold, log10: 3.3–4.3), and in this study, it was also reported that delmopinol (2-[3-(4-propylheptyl)morpholin-4-yl]ethanol) hydrochloride, another amphiphilic compound, even showed higher activity (log10 >5.3). In all cases, the concentration of the active compound was between 0.05 and 0.3 wt%, and the tests were done with contact times of 20 or 30 s. A short overview regarding the use of different agents in mouthwashes in dentistry has recently been given, and one can expect them to be a useful tool in virus prophylaxis [140]. However, still further investigations are needed regarding the efficiency of such approaches, which then could be the basis to develop optimised formulations in that area.
Interestingly, rather mild surfactants like rhamnolipids have also been reported to have marked antiviral activity, e. g., against Herpes Simplex Viruses (HSV), where, as an example, rhamnolipid PS-17 has been shown to be very effective with IC50 values of ~25 μM and a Δlog10 CCID50 of 1.84–2.0 at ~35 μM [142]. The potential of biosurfactants in the fight against COVID-19 has been reviewed very recently, including options for using them as delivery agents [144]. Rhamnolipids are particularly attractive as they are very mild surfactants that are increasingly employed in cosmetic and personal care formulations but also have substantial antimicrobial properties [153,154]. Especially the use of mild surfactants can open up options for employing them in antiviral applications where extended contact times or frequent applications are involved, e.g., mouthwashes, skin lotions, etc., but also in hand washing, since generally surfactants are irritating and therefore total exposure times and concentrations are limited by the irritating or even damaging effect of a given surfactant in such applications. Accordingly, a mild interaction with skin or other body tissue is an attractive feature for surfactants to be applied more generally.
Another type of antiviral surfactant is the anionic peptide surfactant surfactin, which has been shown to be very effective against enveloped viruses and concentrations of 80 μM already result in a reduction of larger than 4.4 log10 for HSV within 15 min [135]. However, at the same time, the applicability of surfactin is severely limited by its cytotoxicity. Accordingly, the synthesis and evaluation of surfactin analogues has been done and was shown to be a promising pathway for developing tailor-made peptide surfactants with antiviral properties and reduced hemolytic activity [155]. Furthermore, other biosurfactants like sophorolipid, a glycolipid produced by yeast, have also been proven to show antiviral activity, e.g., against HIV [141]. Cardiolipin (based on 1,3-bis(sn-3′-phosphatidyl)-sn-glycerol) liposomes act in a more direct way against HIV and can also be employed topically, e.g., intravaginally [156].
Other applications
As discussed above, a major application of surfactants is the disinfection of surfaces. However, they may also be used to impregnate fabrics to inactivate viruses that come in contact with them, which is particularly important if these fabrics are used for personal protection. Recent research has confirmed the antiviral activity of rhamnolipids in that context, and they have been advanced as an additive to mask fabrics with the purpose to inactivate enveloped viruses on a time scale of 3–5 min, thereby potentially significantly enhancing the protection provided by face masks [143]. As stated before, the mildness of rhamnolipids renders them attractive since masks may be carried over extended lengths of time, and therefore, more aggressive compounds could lead to corresponding dermatological problems.
Of course, there is a variety of other applications in which surfactants can be employed in the combat against viruses. An obvious one is in the formulation of antiviral medications. The normal task of the surfactant here is to function as an aiding compound to deliver the drug cargo to an infected cell or to some other places where antiviral activity is needed. A typical surfactant-based drug delivery system is liposomes, which also have been employed successfully for the delivery of antiviral drugs [157]. However, recently it has been shown in studies with cell lines that cationic liposomes based on 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and cholesterol, where the positive charge was introduced by incorporating stearylamine into the lipid bilayer, are quite effective by themselves in blocking the entry of enveloped viruses into cells. This study indicated that the binding of the stearylamine liposomes to the cell membranes is at the origin of the effect [158]. In a somewhat related study, the admixture of polyethyleneimine (PEI) to lecithin liposomes (made from egg phosphatidylcholine) resulted in vesicle formulation (radius: ~150 nm), which performed quite well against HSV [159].
In this context, it might be noted that also more complex surfactant-based systems like nanoemulsions or microemulsions have been shown to be very effective as antiviral formulations, even when rather mild nonionic surfactants like Tween-80 and Span-20 are employed [145]. One reason might be that microemulsions contain a wide range of hydrophilic and hydrophobic components and microenvironments, which may be more effective in interacting with different components of a virus vital for its functioning. For example, the inactivation of the Ebola virus was achieved after only 20 min of treatment with a nanoemulsion containing disinfectant. Ebola virus is known to be relatively resistant to many types of inactivation, and the nanoemulsion treatment provides a mild and effective alternative [120]. The nanoemulsion used for this purpose contained a surfactant mixture of nonionic Triton X-100, anionic tri-n-butyl phosphate (TBP) and cationic cetylpyridinium chloride (CPyC) [121]. Especially CPyC, which is widely employed in personal care products like mouthwashes, has been advanced recently to be a likely candidate for effectively disabling enveloped viruses, including SARS-CoV-2 [122], where its effect is speculated to be a combination of destroying the capsid, as well as lysosomotropic action [123].
Last but not least, we should also mention that surfactants, particularly in the form of lipids, are essential components of delivery vehicles of many vaccines, which applies especially to mRNA-based formulations [146], where typically small vesicles are used, and both natural and synthetic lipids are employed [147]. However, also for the formulation of other COVID-19 vaccine delivery systems, surfactants are widely employed [148]. mRNA needs protection against potential degradation once it is in a biological fluid, and therefore, typically lipid-based vaccine delivery systems are used, and not only in the case of antiviral vaccines [160]. Here pH-sensitive lipids are interesting for controlling mRNA escape from endosomes, and PEG-ylated lipids are important to impart ‘stealth’ properties to the delivery systems. The structure of such lipid nanoparticles as mRNA delivery systems is essential for understanding their performance and has been studied by many methods. In particular, neutron scattering with its unique contrast options has given detailed insights into the internal structure of such lipid nanoparticles (LNPs) [161,162]. While the topic of vaccine delivery is a very interesting one, it is also a very broad topic in itself, and we, therefore, decided not to cover it here in any further detail. But with the help of the given references, the interested reader should be able to find his own way.
Finally, surfactants can also be important in the treatment of the COVID-19 disease. As the disease is mainly affecting the lungs, treatment with lung surfactant can be expected to play an important role in remedying its health effects and potentially contribute to curing the disease [163].
Potential future developments of surfactants against viruses
So far, we discussed applications in which surfactants are either already used or ready for use. However, one may well envision that there are still ample further developments of antiviral applications of surfactants possible, not necessarily always in a direct fashion but also in formulations where surfactants play an important role in rendering this formulation more active by their surface-activity, which typically allows other compounds to become dissolved or to allow accessing areas, where antiviral activity is needed, via their wetting properties.
The upper respiratory tract is a major site for virus replication and transmission, especially in the early stages of infection [164]. It is, therefore, a logical conclusion to try to prevent infection and transmission of the virus with surfactant-containing mouthwashes, sprays, oral rinses, gargles, or even toothpaste. Especially the potential role of oral rinses in targeting the lipid envelope of SARS-CoV-2 has been reviewed recently [165], and certainly, there is a lot of development possible, both in the context of the current pandemic, but also with respect to similar future challenges. In a recent study, several commercially available oral rinses were tested for their activity against SARS-CoV-2. It was found that different SARS-CoV-2 variants can be efficiently inactivated with these oral rinses under biologically relevant conditions [166]. It was suggested in earlier studies already that the antiviral activity of Listerine™ is directed to the viral envelope [167]. In that area, certainly, the use of still milder surfactants might be desirable. One further option for potential applications of surfactant as antiviral agents that has been speculated about [168] is an intranasal application, but that is a topic still to be explored. Quite related in concept to gargles and mouthwashes would be the use of antiviral surfactants in toothpaste, chewing gums or drops, which regularly taken, could provide an additional protective measure against becoming infected.
Another application of different surfactants is the impregnation of protective masks or fabrics for inactivating viruses that come into contact with such fabrics in masks or air filters (in air circulating systems), thereby significantly enhancing their degree of protection.
Potentially surfactants could also play a role not just in preventive measures but also as active compounds in new treatment against the respiratory syndrome caused by SARS-CoV-2. A study showed that the administration of artificial pulmonary surfactant to mice infected with the H1N1 influenza virus in combination with antibody treatment increased the survival rate substantially. The effect was attributed to the surfactant inhibiting alveolar collapse and diffuse alveolar damage in the lungs, thereby preserving lung function for oxygenation [149]. Similarly, phosphatidylglycerol combined with lung surfactant has been advanced for the potential treatment of COVID-19 patients, for instance, with respect to restoring lung surfactant damaged by infection [150]. However, this is beyond the scope of this review that focuses on the interactions between surfactants and viruses, even if it is of great potential importance. One may expect that in the treatment of patients that have suffered from an infection with SARS-CoV-2, surfactants will also play an important role.
Conclusions and outlook
It is evident that surfactants are already a key player in the inhibition of virus infectivity on many levels. Especially enveloped viruses, like SARS-CoV-2, where the surfactants are interacting with and finally dissolving the lipid bilayer, are prone to surfactant-driven deactivation. This has been classically so in the field of disinfection of hard surfaces, skin, hands, fabric, clothes, etc. However, even in these long-established fields of application, one can expect further and extended use of surfactants, especially by employing more effective surfactants and by extending such applications into the range of using mild and ecofriendly surfactants that may come from the area of biosurfactants for instance. Here, rhamnolipids, sophorolipids, and similar compounds hold the most promise. Mild surfactants could also open other avenues of usage, where longer or more frequent exposure of humans is required, as for instance, in mouthwashes, impregnated masks, or fabrics to be worn on the skin. Of course, at the same time, one aims at having as effective surfactants as possible, i.e., working at the lowest possible concentrations, where the guidelines from physical and colloidal chemistry can be applied shrewdly and effectively.
Tailor-made surfactants may work by specifically binding to a given protein, thereby rendering only selected proteins nonfunctional. In that instance, the surfactant would then function like a classical and specific drug. However, also in formulations with drugs, which are not surfactants, one may explore increasingly synergistic effects in delivery, arising from enhanced effectiveness and rate of reducing the infectivity of viruses. Hence, the role of surfactant will likely be dual by helping with delivery but also by contributing more directly to the treatment.
In general, one can safely surmise that much more can be expected with respect to the use of surfactants for antiviral applications. More specifically, unravelling the molecular details of their mode of action will certainly become increasingly important as it would allow specific tailoring of their molecular properties to the particular needs, which would result in increased functional efficiency and efficacy.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Experimental work in the lab of Michael Veit and Klaus Osterrieder is funded by the German Research Foundation (DFG). Miriam Simon thanks the Minerva Stiftung for funding her post-doctoral position.
Glossary
- Activity ai
of a species i means a dimensionless concentration (typically directly related to the mole fraction to which it becomes identical for ideal behaviour, i.e. when intermolecular interactions are absent) that in addition takes into account intermolecular interactions, both with the other molecules of species i, as well as with all other species present in the mixture
- Chemical potential μi
is the partial molar Gibbs free energy (dG/dni)p,T,nj≠iof a given compound i. It determines the thermodynamic potential of this compound and it will attain always the state minimising the μi
- Critical micelle concentration (cmc) / critical aggregate concentration (cac)
is the surfactant concentration above which the formation of micelles, normally of spherical shape, sets in (cmc). However, surfactants with bulkier hydrophobic tails (often double-chain ones, like phospholipids), due to their packing constraints (cylindrical shape), do immediately above this critical concentration form bilayer structures, often vesicles (cac)
- Lipids
in biological membranes are divided into three classes:cholesterol,which is composed of a rigid hydrophobic steroid backbone containing one hydroxyl group as the hydrophilic part,phospholipidsandglycolipids. Glycolipids, as the name indicates, contain a variety of carbohydrates connected to a hydrophobic ceramide moiety containing two usually saturated acyl chains. Phospholipids are composed of glycerol, which is esterified to two fatty acids, mostly one saturated and one unsaturated acyl chain. The third hydroxyl group of glycerol is connected via a phosphate to an alcoholic moiety, either to ethanolamine (the lipid is then called phosphatidyl-ethanolamine, PE), or to choline (PC, also called lecithin), inositol (PI) or serine (PS), which is also an amino acid. Permutations of these different hydrophilic head groups or carbohydrates with fatty acids of different chain length and/or number and location of double bonds creates the very large variety of lipid species. More than 1000 different lipids have been identified in cells and a viral envelope is composed of around 300 different lipid species
- Microemulsion
Thermodynamically stable mixture of oil, water and surfactant, containing nm-sized structural units
- Surfactant
With surfactant we mean in this article: ‘A substance which lowers the surface tension of the medium in which it is dissolved, and/or the interfacial tension with other phases, and, accordingly, is positively adsorbed at the liquid/vapour and/or at other interfaces.’ as defined by IUPAC. This means surface-active agents in the general sense. It might be noted that, different to our use, in the medical literature the term ‘surfactant’ is often used exclusively for lung surfactant (a complex composed of phospholipids and proteins) and detergent for other surface-active compounds
- The virus replication cycle
begins with virus entry, which includes receptor binding followed by release of the viral genome into the cell. Both activities are executed by viral surface (in some casesspike) proteins. In the case of coronaviruses,spikesare large surface projections protruding from the viral membrane. These are transmembrane proteins having a usually short endodomain, which is inside the particle, usually one transmembrane region and a large ectodomain. The genome is then transcribed to generate messenger RNA (mRNA), but in the case of positive-stranded RNA viruses, for example coronaviruses, the genome directly serves as mRNA. The other viral proteins are eithernon-structural proteins, which have a variety of functions, such as genome replication (polymerases) or interfering with the host´s immune response andstructural proteins. The latter assemble with the newly synthesized viral genome to create new virus particles. Enveloped viruses acquire their membrane by pushing through cellular membranes (budding), either at the plasma membrane or at membranes of the exocytic pathway, the ER or the Golgi. In polarized cells, such as epithelial cells, some viruses bud exclusively from theapical membrane, which usually restrict virus infection to the viral entry site; others from thebasolateral membrane, which allows the virus to get access to internal parts of the body. In computer language terms, a virus particle can also be viewed as a vehicle that introduces malicious software, the viral genome, into the cell. The software and the encoded proteins then hijack many of the cell’s functionalities for its own benefit to produce more viral particles, which then spread to neighbouring cells or to a new host. The most efficient viruses are able to induce the generation of up to 3000 new virus particles (also calledvirions) from a single cell within 6 h
This review comes from a themed issue on Hot Topic: COVID-19
Edited by Reinhard Miller and Libero Liggieri
References
- 1.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poon W.C.K., Brown A.T., Direito S.O.L., Hodgson D.J.M., le Nagard L., Lips A., et al. Soft matter science and the COVID-19 pandemic. Soft Matter. 2020;16:8310. doi: 10.1039/d0sm01223h. [DOI] [PubMed] [Google Scholar]
- Yoon B.K., Jeon W.Y., Sut T.N., Cho N.J., Jackman J.A. Stopping membrane-enveloped viruses with nanotechnology strategies: toward antiviral drug development and pandemic preparedness. ACS Nano. 2021;15:125–148. doi: 10.1021/acsnano.0c07489. [DOI] [PubMed] [Google Scholar]; A comprehensive review regarding the options offered by nanotechnology to battle the effects of virus activity.
- 4.Bianculli R.H., Mase J.D., Schulz M.D. Antiviral polymers: past approaches and future possibilities. Macromolecules. 2020;53:9158–9186. doi: 10.1021/acs.macromol.0c01273. [DOI] [Google Scholar]
- 5.Lombardi M.E., Ladman B.S., Alphin R.L., Benson E.R. Inactivation of avian influenza virus using common detergents and chemicals. Avian Dis. 2008;52:118–123. doi: 10.1637/8055-070907-Reg. [DOI] [PubMed] [Google Scholar]
- 6.Doultree J.C., Druce J.D., Birch C.J., Bowden D.S., Marshall J.A. Inactivation of feline calicivirus, a Norwalk virus surrogate. J Hosp Infect. 1999;41:51–57. doi: 10.1016/S0195-6701(99)90037-3. [DOI] [PubMed] [Google Scholar]
- 7.Falk N.A. Surfactants as antimicrobials: a brief overview of microbial interfacial chemistry and surfactant antimicrobial activity. J Surfactants Deterg. 2019;22:1119–1127. doi: 10.1002/jsde.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown D.A., London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 9.Levental I., Levental K.R., Heberle F.A. Lipid rafts: controversies resolved, mysteries remain. Trends Cell Biol. 2020;30:341–353. doi: 10.1016/j.tcb.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumgart T., Hess S.T., Webb W.W. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 2003;425:821–824. doi: 10.1038/nature02013. [DOI] [PubMed] [Google Scholar]
- 11.Honigmann A., Mueller V., Hell S.W., Eggeling C. STED microscopy detects and quantifies liquid phase separation in lipid membranes using a new far-red emitting fluorescent phosphoglycerolipid analogue. Faraday Discuss. 2013;161:77–89. doi: 10.1039/c2fd20107k. [DOI] [PubMed] [Google Scholar]
- 12.Moon S., Yan R., Kenny S.J., Shyu Y., Xiang L., Li W., et al. Spectrally resolved, functional super-resolution microscopy reveals nanoscale compositional heterogeneity in live-cell membranes. J Am Chem Soc. 2017;139:10944–10947. doi: 10.1021/jacs.7b03846. [DOI] [PubMed] [Google Scholar]
- 13.Connell S.D., Smith D.A. The atomic force microscope as a tool for studying phase separation in lipid membranes (review) Mol Membr Biol. 2006;23:17–28. doi: 10.1080/09687860500501158. [DOI] [PubMed] [Google Scholar]
- 14.Zhong J. From simple to complex: investigating the effects of lipid composition and phase on the membrane interactions of biomolecules using in situ atomic force microscopy. Integr Biol. 2011;3:632–644. doi: 10.1039/c0ib00157k. [DOI] [PubMed] [Google Scholar]
- 15.Raghunathan K., Kenworthy A.K. Dynamic pattern generation in cell membranes: current insights into membrane organization. Biochim Biophys Acta. 2018;1860:2018–2031. doi: 10.1016/j.bbamem.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owen D.M., Gaus K. Imaging lipid domains in cell membranes: the advent of super-resolution fluorescence microscopy. Front Plant Sci. 2013;4:503. doi: 10.3389/fpls.2013.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kusumi A., Fujiwara T.K., Tsunoyama T.A., Kasai R.S., Liu A.A., Hirosawa K.M., et al. Defining raft domains in the plasma membrane. Traffic. 2020;21:106–137. doi: 10.1111/tra.12718. [DOI] [PubMed] [Google Scholar]
- 18.Simons K., Gerl M.J. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 19.Hanson M.A., Cherezov V., Griffith M.T., Roth C.B., Jaakola V.P., Chien E.Y.T., et al. A specific cholesterol binding site is established by the 2.8 Å structure of the human β2-adrenergic receptor. Structure. 2008;16:897–905. doi: 10.1016/j.str.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu B., Höfer C.T., Thiele C., Veit M. Cholesterol binding to the transmembrane region of a group 2 hemagglutinin (HA) of influenza virus is essential for virus replication, affecting both virus assembly and HA fusion activity. J Virol. 2019;93 doi: 10.1128/jvi.00555-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J., Kreutzberger A.J.B., Odongo L., Nelson E.A., Nyenhuis D.A., Kiessling V., et al. Ebola virus glycoprotein interacts with cholesterol to enhance membrane fusion and cell entry. Nat Struct Mol Biol. 2021;28:181–189. doi: 10.1038/s41594-020-00548-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders D.W., Jumper C.C., Ackerman P.J., Bracha D., Donlic A., Kim H., et al. Sars-cov-2 requires cholesterol for viral entry and pathological syncytia formation. eLife. 2021;10 doi: 10.7554/ELIFE.65962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNunno N.M., Goetschius D.J., Narayanan A., Majowicz S.A., Moustafa I., Bator C.M., et al. Identification of a pocket factor that is critical to Zika virus assembly. Nat Commun. 2020;11:1–8. doi: 10.1038/s41467-020-18747-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with references 17–19, it is demonstrated that the non-covalent binding of lipids (cholesterol or fatty acids) to short amino acid motifs is a common feature of viral glycoproteins. Since binding is functionally important for cell entry and/or assembly of viral particles it is a potential target of surfactants.
- Toelzer C., Gupta K., Yadav S.K.N., Borucu U., Davidson A.D., Williamson M.K., et al. Free fatty acid binding pocket in the locked structure of SARS-CoV-2 spike protein. Science. 2020;370:725–730. doi: 10.1126/science.abd3255. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article is very interesting as it discusses the specific interactions of a simple fatty acid with the spike protein of SARS-CoV-2 and how it reduces the effectiveness of this protein in virus infectivity. Such molecularly resolved information of rather simple surfactant molecules with respect to affecting virus activity should become increasingly important for future advanced formulations using surfactants to control virus replication.
- 25.Wu J., Chan R., Wenk M.R., Hew C.L. Lipidomic study of intracellular Singapore grouper iridovirus. Virology. 2010;399:248–256. doi: 10.1016/j.virol.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmerberg J., Kozlov M.M. How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol. 2006;7:9–19. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]
- 27.Israelachvili J.N., Mitchell D.J., Ninham B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J Chem Soc, Faraday Trans 2. 1976;72:1525–1568. doi: 10.1039/F29767201525. [DOI] [Google Scholar]
- van Meer G., Voelker D.R., Feigenson G.W. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]; Classical and easy to read review article that introduces newcomers to the field of cell biology of lipids
- 29.Lorent J.H., Levental K.R., Ganesan L., Rivera-Longsworth G., Sezgin E., Doktorova M., et al. Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat Chem Biol. 2020;16:644–652. doi: 10.1038/s41589-020-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flint J., Racaniello V.R., Rall G.F., Hatziioannou T., Skalka A.M. 5th ed. vol. 1. John Wiley & Sons, Inc.; 2020. Principles of virology. (Molecular biology). [Google Scholar]
- 31.Ke Z., Oton J., Qu K., Cortese M., Zila V., McKeane L., et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature. 2020;588:498–502. doi: 10.1038/s41586-020-2665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luque D., Castón J.R. Cryo-electron microscopy for the study of virus assembly. Nat Chem Biol. 2020;16:231–239. doi: 10.1038/s41589-020-0477-1. [DOI] [PubMed] [Google Scholar]
- 33.Walls A., Tortorici M.A., Bosch B.J., Br Frenz, Rottier P.J.M., DiMaio F., et al. Crucial steps in the structure determination of a coronavirus spike glycoprotein using cryo-electron microscopy. Protein Sci. 2017;26:113–121. doi: 10.1002/pro.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bar-on Y.M., Flamholz A., Phillips R., Milo R. SARS-CoV-2 (COVID-19) by the numbers. eLife. 2020;9 doi: 10.7554/eLife.57309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neuman B.W., Kiss G., Kunding A.H., Bhella D., Baksh M.F., Connelly S., et al. A structural analysis of M protein in coronavirus assembly and morphology. J Struct Biol. 2011;174:11–22. doi: 10.1016/j.jsb.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McBride R., van Zyl M., Fielding B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6:2991–3018. doi: 10.3390/v6082991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan X., Cao D., Kong L., Zhang X. Cryo-EM analysis of the post-fusion structure of the SARS-CoV spike glycoprotein. Nat Commun. 2020;11:3618. doi: 10.1038/s41467-020-17371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann M., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turoňová B., Sikora M., Schürmann C., Hagen W.J.H., Welsch S., Blanc F.E.C., et al. In situ structural analysis of SARS-CoV-2 spike reveals flexibility mediated by three hinges. Science. 2020;370:203–208. doi: 10.1126/science.abd5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein S., Cortese M., Winter S.L., Wachsmuth-Melm M., Neufeldt C.J., Cerikan B., et al. SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography. Nat Commun. 2020;11:5885. doi: 10.1038/s41467-020-19619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klenk H.D. Springer; Berlin, Heidelberg: 1974. Viral envelopes and their relationship to cellular membranes. [DOI] [PubMed] [Google Scholar]
- 42.Kalvodova L., Sampaio J.L., Cordo S., Ejsing C.S., Shevchenko A., Simons K. The lipidomes of vesicular stomatitis virus, Semliki forest virus, and the host plasma membrane analyzed by quantitative shotgun mass spectrometry. J Virol. 2009;83:7996–8003. doi: 10.1128/jvi.00635-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerl M.J., Sampaio J.L., Urban S., Kalvodova L., Verbavatz J.M., Binnington B., et al. Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane. J Cell Biol. 2012;196:213–221. doi: 10.1083/jcb.201108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brügger B., Glass B., Haberkant P., Leibrecht I., Wieland F.T., Kräusslich H.G. The HIV lipidome: a raft with an unusual composition. Proc Natl Acad Sci USA. 2006;103:2641–2646. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]; First detailed analysis of the lipid composition of an enveloped virus particle using advanced lipidomics methods.
- 45.Merz A., Long G., Hiet M.S., Brügger B., Chlanda P., Andre P., et al. Biochemical and morphological properties of hepatitis C virus particles and determination of their lipidome. J Biol Chem. 2011;286:3018–3032. doi: 10.1074/jbc.M110.175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Callens N., Brügger B., Bonnafous P., Drobecq H., Gerl M.J., Krey T., et al. Morphology and molecular composition of purified bovine viral Diarrhea virus envelope. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Genderen I.L., Godeke G.J., Rottier P.J.M., van Meer G. The phospholipid composition of enveloped viruses depends on the intracellular membrane through which they bud. Biochem Soc Trans. 1995;23:523–526. doi: 10.1042/bst0230523. [DOI] [PubMed] [Google Scholar]
- 48.Chakraborty S., Doktorova M., Molugu T.R., Heberle F.A., Scott H.L., Dzikovski B., et al. How cholesterol stiffens unsaturated lipid membranes. Proc Natl Acad Sci USA. 2020;117:21896–21905. doi: 10.1073/pnas.2004807117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reddy T., Sansom M.S.P. The role of the membrane in the structure and biophysical robustness of the Dengue virion envelope. Structure. 2016;24:375–382. doi: 10.1016/j.str.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li S., Eghiaian F., Sieben C., Herrmann A., Schaap I.A.T. Bending and puncturing the influenza lipid envelope. Biophys J. 2011;100:637–645. doi: 10.1016/j.bpj.2010.12.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaap I.A.T., Eghiaia F., des George A., Veigel C. Effect of envelope proteins on the mechanical properties of influenza virus. J Biol Chem. 2012;287:41078–41088. doi: 10.1074/jbc.M112.412726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polozov I.V., Bezrukov L., Gawrisch K., Zimmerberg J. Progressive ordering with decreasing temperature of the phospholipids of influenza virus. Nat Chem Biol. 2008;4:248–255. doi: 10.1038/nchembio.77. [DOI] [PubMed] [Google Scholar]
- Zandi R., Dragnea B., Travesset A., Podgornik R. On virus growth and form. Phys Rep. 2020;847:1–102. doi: 10.1016/j.physrep.2019.12.005. [DOI] [Google Scholar]; An article directly from the ‘pre-Corona time’ that discusses in comprehensive detail the various aspects, how a virus can be seen from the physicists’ point, which includes classification of the form, nanomechanics, the growth/assembly process, as well as electrostatics and genome packaging. Clearly to be recommended for anyone interesting in the physics of viruses.
- 54.Šiber A., Božič A.L., Podgornik R. Energies and pressures in viruses: contribution of nonspecific electrostatic interactions. Phys Chem Chem Phys. 2012;14:3746–3765. doi: 10.1039/c1cp22756d. [DOI] [PubMed] [Google Scholar]
- 55.Bruinsma R.F., Comas-Garcia M., Garmann R.F., Grosberg A.Y. Equilibrium self-assembly of small RNA viruses. Phys Rev E. 2016;93 doi: 10.1103/PhysRevE.93.032405. [DOI] [PubMed] [Google Scholar]
- 56.Hernando-Pérez M., Cartagena-Rivera A.X., Lošdorfer Božič A., Carrillo P.J.P., San Martín C., Mateu M.G., et al. Quantitative nanoscale electrostatics of viruses. Nanoscale. 2015;7:17289–17298. doi: 10.1039/c5nr04274g. [DOI] [PubMed] [Google Scholar]
- Cadena-Nava R.D., Comas-Garcia M., Garmann R.F., Rao A.L.N., Knobler C.M., Gelbart W.M. Self-assembly of viral capsid protein and RNA molecules of different sizes: requirement for a specific high protein/RNA mass ratio. J Virol. 2012;86:3318–3326. doi: 10.1128/jvi.06566-11. [DOI] [PMC free article] [PubMed] [Google Scholar]; A thorough account of the basic principles that govern self-assembly of viral capsids, an essential aspect of virus replication.
- 58.Caspar D.L., Klug A. Physical principles in the construction of regular viruses. Cold Spring Harbor Symp Quant Biol. 1962;27:1–24. doi: 10.1101/SQB.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- 59.Montiel-Garcia D, Santoyo-Rivera N, Ho P, Carrillo-Tripp M, Johnson JE, Reddy VS. VIPERdb v3. 0: a structure-based data analytics platform for viral capsids n.d. http://viperdb.scripps.edu/(accessed May 24, 2021). [DOI] [PMC free article] [PubMed]
- 60.Garmann R.F., Goldfain A.M., Manoharan V.N. Measurements of the self-assembly kinetics of individual viral capsids around their RNA genome. Proc Natl Acad Sci USA. 2019;116:22485–22490. doi: 10.1073/pnas.1909223116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arranz R., Coloma R., Chichón F.J., Conesa J.J., Carrascosa J.L., Valpuesta J.M., et al. The structure of native influenza virion ribonucleoproteins. Science. 2012;338:1634–1637. doi: 10.1126/science.1228172. [DOI] [PubMed] [Google Scholar]
- 62.Tan A., Ziegler A., Steinbauer B., Seelig J. Thermodynamics of sodium dodecyl sulfate partitioning into lipid membranes. Biophys J. 2002;83:1547–1556. doi: 10.1016/S0006-3495(02)73924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richieri G.V., Ogata R.T., Kleinfeld A.M. Thermodynamics of fatty acid binding to fatty acid-binding proteins and fatty acid partition between water and membranes measured using the fluorescent probe ADIFAB. J Biol Chem. 1995;270:P15076–P15084. doi: 10.1074/jbc.270.25.15076. [DOI] [PubMed] [Google Scholar]
- 64.Rosen M.J., Kunjappu J.T. 4th ed. 2012. Surfactants and interfacial phenomena. [DOI] [Google Scholar]
- 65.Murphy D.S. Fabric softener technology: a review. J Surfactants Deterg. 2015;18:199–204. doi: 10.1007/s11743-014-1658-2. [DOI] [Google Scholar]
- 66.Burkhardt M., Martinez-Castro N., Tea S., Drechsler M., Babin I., Grishagin I., et al. Polyisobutylene-block-poly(methacrylic acid) diblock copolymers: self-assembly in aqueous media. Langmuir. 2007;23:12864–12874. doi: 10.1021/la701807b. [DOI] [PubMed] [Google Scholar]
- 67.Demina T., Grozdova I., Krylova O., Zhirnov A., Istratov V., Frey H., et al. Relationship between the structure of amphiphilic copolymers and their ability to disturb lipid bilayers. Biochemistry. 2005;44:4042–4054. doi: 10.1021/bi048373q. [DOI] [PubMed] [Google Scholar]
- 68.Heerklotz H., Seelig J. Titration calorimetry of surfactant-membrane partitioning and membrane solubilization. Biochim Biophys Acta. 2000;1508:69–85. doi: 10.1016/S0304-4157(00)00009-5. [DOI] [PubMed] [Google Scholar]
- 69.Timmer N., Droge S.T.J. Sorption of cationic surfactants to artificial cell membranes: comparing phospholipid bilayers with monolayer coatings and molecular simulations. Environ Sci Technol. 2017;51:2890–2898. doi: 10.1021/acs.est.6b05662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara T., Akiba I., Sakou M., Sakaguchi T., Taniguchi H. Inactivation of human and avian influenza viruses by potassium oleate of natural soap component through exothermic interaction. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204908. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper gives sound experimental details on how simple soaps like potassium oleate interact with viruses, and thereby, are able to disenable their function. More fundamental thermodynamic investigations of this sort will help to clarify further the aspects relevant to suppress virus activity by simple detergents.
- 71.Smoluchowski M.v. Versuch einer mathematischen Theorie der Koagulationskinetik kolloider Lösungen. Z Phys Chem. 1917;92:129–168. doi: 10.1515/zpch-1918-9209. [DOI] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975;415:29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]; Classic review article on the use of detergents to extract glycoproteins in functional form from membranes. This allowed viral glycoproteins to be studied by biochemical methods.
- 73.Montalvo G., Khan A. Self-assembly of mixed ionic and zwitterionic amphiphiles: associative and dissociative interactions between lamellar phases. Langmuir. 2002;18:8330–8339. doi: 10.1021/la0204489. [DOI] [Google Scholar]
- 74.Bilalov A., Olsson U., Lindman B. DNA-lipid self-assembly: phase behavior and phase structures of a DNA-surfactant complex mixed with lecithin and water. Soft Matter. 2011;7:730–742. doi: 10.1039/c0sm00650e. [DOI] [Google Scholar]
- 75.Sadaghiani A.S., Khan A., Lindman B. Liquid crystallinity of lecithin systems. Ternary phase diagrams of lecithin-water with triton X-100 and decanol. J Colloid Interface Sci. 1989;132:352–362. doi: 10.1016/0021-9797(89)90250-6. [DOI] [Google Scholar]
- 76.Small D.M. A classification of biologic lipids based upon their interaction in aqueous systems. J Am Oil Chem Soc. 1968;45:108–119. doi: 10.1007/BF02915334. [DOI] [PubMed] [Google Scholar]
- 77.Dennis E.A., Owens J.M. Studies on mixed micelles of triton X-100 and phosphatidylcholine using nuclear magnetic resonance techniques. J Supramol Struct. 1973;1:165–176. doi: 10.1002/jss.400010302. [DOI] [PubMed] [Google Scholar]
- 78.Goñi F.M., Urbaneja M.-A., Arrondo J.L., Alonso A., Durrani A.A., Chapman D. The interaction of phosphatidylcholine bilayers with Triton X-100. Eur J Biochem. 1986;160:659–665. doi: 10.1111/j.1432-1033.1986.tb10088.x. [DOI] [PubMed] [Google Scholar]
- Walter A., Vinson P.K., Kaplun A., Talmon Y. Intermediate structures in the cholate-phosphatidylcholine vesicle-micelle transition. Biophys J. 1991;60:1315–1325. doi: 10.1016/S0006-3495(91)82169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive cryo-TEM study of PC vesicles with increasing cholate concentration, visualizing the steps of membrane disintegration described by Helenius and Simons.
- 80.Edwards K., Almgren M. Solubilization of lecithin vesicles by C12E8. Structural transitions and temperature effects. J Colloid Interface Sci. 1991;14:1–21. doi: 10.1016/0021-9797(91)90129-V. [DOI] [Google Scholar]
- Almgren M. Mixed micelles and other structures in the solubilization of bilayer lipid membranes by surfactants. Biochim Biophys Acta. 2000;1508:146–163. doi: 10.1016/S0005-2736(00)00309-6. [DOI] [PubMed] [Google Scholar]; A comprehensive review regarding the effect of surfactant addition on lipid bilayers from the experimentalist’s point of view, with an emphasis on using cryo-EM to depict the intermediate structures formed.
- 82.Babnik Bl, Miklavčič D., Kandušer M., Hägerstrand H., Kralj-Iglič V., Iglič A. Shape transformation and burst of giant POPC unilamellar liposomes modulated by non-ionic detergent C12E8. Chem Phys Lipids. 2003;125:123–138. doi: 10.1016/S0009-3084(03)00084-7. [DOI] [PubMed] [Google Scholar]
- 83.Stuart M.C.A., Boekema E.J. Two distinct mechanisms of vesicle-to-micelle and micelle-to-vesicle transition are mediated by the packing parameter of phospholipid-detergent systems. Biochim Biophys Acta. 2007;1768:2681–2689. doi: 10.1016/j.bbamem.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Heerklotz H. Interactions of surfactants with lipid membranes. Q Rev Biophys. 2008;41:205–264. doi: 10.1017/S0033583508004721. [DOI] [PubMed] [Google Scholar]; A very thorough and comprehensive review regarding the different relevant aspects of interactions of surfactant with lipid bilayers. A strong focus is on thermodynamics, but also other aspects like kinetics and applications of such interactions are covered
- 85.Nazari M., Kurdi M., Heerklotz H. Classifying surfactants with respect to their effect on lipid membrane order. Biophys J. 2012;102:498–506. doi: 10.1016/j.bpj.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin D., Grossfield A. Thermodynamics of antimicrobial lipopeptide binding to membranes: origins of affinity and selectivity. Biophys J. 2014;107:1862–1872. doi: 10.1016/j.bpj.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lichtenberg D., Ahyayauch H., Goñi F.M. The mechanism of detergent solubilization of lipid bilayers. Biophys J. 2013;105:289–299. doi: 10.1016/j.bpj.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cócera M., López O., Pons R., Amenitsch H., de La Maza A. Effect of the electrostatic charge on the mechanism inducing liposome solubilization: a kinetic study by synchrotron radiation SAXS. Langmuir. 2004;20:3074–3079. doi: 10.1021/la035972+. [DOI] [PubMed] [Google Scholar]
- 89.Otzen D. Protein-surfactant interactions: a tale of many states. Biochim Biophys Acta. 2011;1814:562–591. doi: 10.1016/j.bbapap.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 90.Stenstam A., Montalvo G., Grillo I., Gradzielski M. Small angle neutron scattering study of Lysozyme-Sodium dodecyl sulfate aggregates. J Phys Chem B. 2003;107:12331–12338. doi: 10.1021/jp0352783. [DOI] [Google Scholar]
- 91.Richieri G.V., Ogata R.T., Zimmerman A.W., Veerkamp J.H., Kleinfeld A.M. Fatty acid binding proteins from different tissues show distinct patterns of fatty acid interactions. Biochemistry. 2000;39:7197–7204. doi: 10.1021/bi000314z. [DOI] [PubMed] [Google Scholar]
- 92.Tanford C. 2nd ed. John Wiley & Sons; 1981. The hydrophobic effect: formation of micelles and biological membranes. [Google Scholar]
- 93.Bojesen E., Bojesen I.N. Albumin binding of long-chain fatty acids: thermodynamics and kinetics. J Phys Chem. 1996;100:17981–17985. doi: 10.1021/jp962141m. [DOI] [Google Scholar]
- 94.Richieri G.Y., Ogata R.T., Kleinfeld A.M. Thermodynamic and kinetic properties of fatty acid interactions with rat liver fatty acid-binding protein. J Biol Chem. 1996;271:31068–31074. doi: 10.1074/jbc.271.49.31068. [DOI] [PubMed] [Google Scholar]
- 95.Ivanova P.T., Myers D.S., Milne S.B., McClaren J.L., Thomas P.G., Brown H.A. Lipid composition of the viral envelope of three strains of influenza virus - not all viruses are created equal. ACS Infect Dis. 2015;1:435–442. doi: 10.1021/acsinfecdis.5b00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kragh-Hansen U., le Maire M., Møller J.V. The mechanism of detergent solubilization of liposomes and protein- containing membranes. Biophys J. 1998;75:2932–2946. doi: 10.1016/S0006-3495(98)77735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R., Helenius A., Simons K. Solubilization of the Semliki forest virus membrane with sodium dodecyl sulfate. Biochemistry. 1975;14:1835–1841. doi: 10.1021/bi00680a005. [DOI] [PubMed] [Google Scholar]; A nice study, following the solubilization of Semliki Forest Virus upon addition of the anionic surfactant SDS by density gradient centrifugation.
- 98.Laine R., Söderlund H., Renkonen O. Chemical composition of semiliki forest virus. Intervirology. 1973;1:110–118. doi: 10.1159/000148837. [DOI] [PubMed] [Google Scholar]
- 99.Simons K., Helenius A., Garoff H. Solubilization of the membrane proteins from Semliki forest virus with triton X100. J Mol Biol. 1973;80:119–133. doi: 10.1016/0022-2836(73)90236-2. [DOI] [PubMed] [Google Scholar]
- 100.Sturman L.S., Ricard C.S., Holmes K.V. Conformational change of the coronavirus peplomer glycoprotein at pH 8.0 and 37°C correlates with virus aggregation and virus-induced cell fusion. J Virol. 1990;64:3042–3050. doi: 10.1128/jvi.64.6.3042-3050.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zawada K.E., Wrona D., Rawle R.J., Kasson P.M. Influenza viral membrane fusion is sensitive to sterol concentration but surprisingly robust to sterol chemical identity. Sci Rep. 2016;6:1–10. doi: 10.1038/srep29842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]; This recent work gives a comprehensive compilation of data available regarding the effectiveness of a larger variety of surfactants and other antiviral agents in disinfection with respect to coronaviruses.
- 103.Jahromi R., Mogharab V., Jahromi H., Avazpour A. Synergistic effects of anionic surfactants on coronavirus (SARS-CoV-2) virucidal efficiency of sanitizing fluids to fight COVID-19. Food Chem Toxicol. 2020;145 doi: 10.1016/j.fct.2020.111702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin Q., Lim J.Y.C., Xue K., Yew P.Y.M., Owh C., Chee P.L., et al. Sanitizing agents for virus inactivation and disinfection. View. 2020;1:e16. doi: 10.1002/viw2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wolff M.H., Sattar S.A., Adegbunrin O., Tetro J. Birkhäuser Basel; 2005. Environmental survival and microbicide inactivation of coronaviruses. Coronaviruses with special emphasis on first insights concerning SARS; pp. 201–212. [DOI] [Google Scholar]
- Springthorpe V.S., Sattar S.A. Chemical disinfection of virus-contaminated surfaces. Crit Rev Environ Contr. 1990;20:169–229. doi: 10.1080/10643389009388396. [DOI] [Google Scholar]; A classical review on chemical disinfection of surfaces with a marked focus on eliminating virus activity. Despite its age, this review, with its very comprehensive discussion, the various relevant parameters and aspects, is very suitable to gain a general insight into the field of surface disinfection.
- Anestopoulos I., Kiousi D.E., Klavaris A., Galanis A., Salek K., Euston S.R., et al. Surface active agents and their health-promoting properties: molecules of multifunctional significance. Pharmaceutics. 2020;12:688. doi: 10.3390/pharmaceutics12070688. [DOI] [PMC free article] [PubMed] [Google Scholar]; A very recent and comprehensive review regarding the use of surfactants for promoting the health state of humans with a focus on the microbial and antiviral properties of surfactants. A particular focus is given on newer developments in the field and the increasing use of biosurfactants in it.
- 108.Thurman R.B., Gerba C.P. Molecular mechanisms of viral inactivation by water disinfectants. Adv Appl Microbiol. 1988;33:75–105. doi: 10.1016/S0065-2164(08)70205-3. [DOI] [PubMed] [Google Scholar]
- 109.Stock C.C., Francis T. The inactivation of the virus of epidemic influenza by soaps. J Exp Med. 1940;71:661–682. doi: 10.1084/jem.71.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bawden F.C., Pirie N.W. Liquid crystalline preparations of potoato virus “X”. Br J Exp Pathol. 1938;19:66–82. [Google Scholar]
- 111.Piret J., Desormeaux A., Bergeron M.G. Sodium lauryl sulfate, a microbicide effective against enveloped and nonenveloped viruses. Curr Drug Targets. 2002;3:17–30. doi: 10.2174/1389450023348037. [DOI] [PubMed] [Google Scholar]
- 112.Geller C., Varbanov M., Duval R. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses. 2012;4:3044–3068. doi: 10.3390/v4113044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu P., Yuen Y., Hsiao H.M., Jaykus L.A., Moe C. Effectiveness of liquid soap and hand sanitizer against Norwalk virus on contaminated hands. Appl Environ Microbiol. 2010;76:394–399. doi: 10.1128/AEM.01729-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wood A., Payne D. The action of three antiseptics/disinfectants against enveloped and non-enveloped viruses. J Hosp Infect. 1998;38:283–295. doi: 10.1016/S0195-6701(98)90077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van Hemert M.J., van den Worm S.H.E., Knoops K., Mommaas A.M., Gorbalenya A.E., Snijder E.J. SARS-coronavirus replication/transcription complexes are membrane-protected and need a host factor for activity in vitro. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microb. 2020;1:e10. doi: 10.1016/s2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gerlach M., Wolff S., Ludwig S., Schäfer W., Keiner B., Roth N.J., et al. Rapid SARS-CoV-2 inactivation by commonly available chemicals on inanimate surfaces. J Hosp Infect. 2020;106:633–634. doi: 10.1016/j.jhin.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saknimit M., Inatsuki I., Sugiyama Y., Yagami K. Virucidal efficacy of physico-chemical treatments against coronaviruses and parvoviruses of laboratory animals. Jikken Dobutsu. 1988;37:341–345. doi: 10.1538/expanim1978.37.3_341. [DOI] [PubMed] [Google Scholar]
- 119.Pratelli A. Action of disinfectants on canine coronavirus replication in vitro. Zoonoses Public Health. 2007;54:383–386. doi: 10.1111/j.1863-2378.2007.01079.x. [DOI] [PubMed] [Google Scholar]
- 120.Chepurnov A.A., Bakulina L.F., Dadaeva A.A., Ustinova E.N., Chepurnova T.S., Baker J.R. Inactivation of Ebola virus with a surfactant nanoemulsion. Acta Trop. 2003;87:315–320. doi: 10.1016/S0001-706X(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 121.Baker J.R., Jr., Hamouda T., Shih A., Myc A. 2012. Antimicrobial nanoemulsion compositions and methods. Patent US20090143476A1. [Google Scholar]
- 122.Disinfectant Concentrations, Contact Times, and Use Settings for EPA's List of Products Effective against SARS-CoV-2, the Cause of COVID-19 n.d. https://www.ecri.org/components/HDJournal/Pages/Disinfectant-Concentrations-for-EPA-list-N-COVID-19.aspx (accessed February 15, 2021).
- 123.Baker N., Williams A.J., Tropsha A., Ekins S. Repurposing quaternary ammonium compounds as potential treatments for COVID-19. Pharm Res (N Y) 2020;37:104. doi: 10.1007/s11095-020-02842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Springthorpe V.S., Grenier J.L., Lloyd-Evans N., Sattar S.A. Chemical disinfection of human rotaviruses: efficacy of commercially-available products in suspension tests. J Hyg. 1986;97:139–161. doi: 10.1017/S0022172400064433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sattar S.A., Springthorpe V.S., Karim Y., Loro P. Chemical disinfection of non-porous inanimate surfaces experimentally contaminated with four human pathogenic viruses. Epidemiol Infect. 1989;102:493–505. doi: 10.1017/S0950268800030211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schrank C.L., Minbiole K.P.C., Wuest W.M. Are quaternary ammonium compounds, the workhorse disinfectants, effective against severe acute respiratory syndrome-coronavirus-2? ACS Infect Dis. 2020;6:1553–1557. doi: 10.1021/acsinfecdis.0c00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hora P.I., Pati S.G., McNamara P.J., Arnold W.A. Increased use of quaternary ammonium compounds during the SARS-CoV-2 pandemic and beyond: consideration of environmental implications. Environ Sci Technol Lett. 2020;7:622–631. doi: 10.1021/acs.estlett.0c00437. [DOI] [PubMed] [Google Scholar]
- 128.Hakimi A.A., Armstrong W.B. Hand sanitizer in a pandemic: wrong formulations in the wrong hands. J Emerg Med. 2020;59:668–672. doi: 10.1016/j.jemermed.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087–1088. doi: 10.1016/j.jaad.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rundle C.W., Presley C.L., Militello M., Barber C., Powell D.L., Jacob S.E., et al. Hand hygiene during COVID-19: recommendations from the American contact dermatitis society. J Am Acad Dermatol. 2020;83:1730–1737. doi: 10.1016/j.jaad.2020.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Leclercq L., Nardello-Rataj V. How to improve the chemical disinfection of contaminated surfaces by viruses, bacteria and fungus? Eur J Pharmaceut Sci. 2020;155:105559. doi: 10.1016/j.ejps.2020.105559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hard-surface disinfectants and hand sanitizers (COVID-19): List of disinfectants with evidence for use against COVID-19 n.d. https://www.canada.ca/en/health-canada/services/drugs-health-products/disinfectants/covid-19/list.html (accessed May 19, 2021).
- 133.Smith M.L., Gandolfi S., Coshall P.M., Rahman P.K.S.M. Biosurfactants: a covid-19 perspective. Front Microbiol. 2020;11:1341. doi: 10.3389/fmicb.2020.01341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yuan L., Zhang S., Wang Y., Li Y., Wang X., Yang Q. Surfactin inhibits membrane fusion during invasion of epithelial cells by enveloped viruses. J Virol. 2018;92 doi: 10.1128/jvi.00809-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vollenbroich D., Özel M., Vater J., Kamp R.M., Pauli G. Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from Bacillus subtilis. Biologicals. 1997;25:289–297. doi: 10.1006/biol.1997.0099. [DOI] [PubMed] [Google Scholar]
- 136.Borsanyiova M., Patil A., Mukherji R., Prabhune A., Bopegamage S. Biological activity of sophorolipids and their possible use as antiviral agents. Folia Microbiol. 2016;61:85–89. doi: 10.1007/s12223-015-0413-z. [DOI] [PubMed] [Google Scholar]
- 137.Cheng P.W., Ng L.T., Chiang L.C., Lin C.C. Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clin Exp Pharmacol Physiol. 2006;33:612–616. doi: 10.1111/j.1440-1681.2006.04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz H., Mendenhall M. Comparative analysis of antiviral efficacy of four different mouthwashes against severe acute respiratory syndrome coronavirus 2: an in vitro study. Int J Exp Dent Sci. 2020;9:1–3. doi: 10.5005/jp-journals-10029-1209. [DOI] [Google Scholar]; Interesting article regarding a potentially important application of surfactants against virus activity by using mouthwashes/gargles. This application could open up to interesting future formulations in the field.
- 139.Komine A., Yamaguchi E., Okamoto N., Yamamoto K. Virucidal activity of oral care products against SARS-CoV-2 in vitro. J Oral and Maxillofac Surg Med Pathol. July 2021;33:475–477. doi: 10.1016/j.ajoms.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vergara-Buenaventura A., Castro-Ruiz C. Use of mouthwashes against COVID-19 in dentistry. Br J Oral Maxillofac Surg. 2020;58:924–927. doi: 10.1016/j.bjoms.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shah V., Doncel G.F., Seyoum T., Eaton K.M., Zalenskaya I., Hagver R., et al. Sophorolipids, microbial glycolipids with anti-human immunodeficiency virus and sperm-immobilizing activities. Antimicrob Agents Chemother. 2005;49:4093–4100. doi: 10.1128/AAC.49.10.4093-4100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Remichkova M., Galabova D., Roeva I., Karpenko E., Shulga A., Galabov A.S. Anti-herpesvirus activities of Pseudomonas sp. S-17 rhamnolipid and its complex with alginate. Z Naturforsch. 2008;63:75–81. doi: 10.1515/znc-2008-1-214. [DOI] [PubMed] [Google Scholar]
- Jin L., Black W., Sawyer T. 2020. Characterization of rhamnolipids for the inactivation of enveloped viruses. [DOI] [Google Scholar]; A study that shows how cationic and nonionic surfactant, together with cyclodextrin, can work synergistically as a disinfectant. Here, in particular, the relations to the physicochemical parameters of the system are dwelled on, and the composition of the ternary disinfectant mixture is systematically studied.
- 144.Çelik A., Manga E.B., Çabuk A., Banat I.M. Biosurfactants' potential role in combating covid-19 and similar future microbial threats. Appl Sci. 2021;11:334. doi: 10.3390/app11010334. [DOI] [Google Scholar]
- 145.Alkhatib M.H., Aly M.M., Rahbeni R.A., Balamash K.S. Antimicrobial activity of biocompatible microemulsions against Aspergillus Niger and herpes simplex virus type 2. Jundishapur J Microbiol. 2016;9 doi: 10.5812/jjm.37437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Reichmuth A.M., Oberli M.A., Jeklenec A., Langer R., Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther Deliv. 2016;7:319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Buschmann M.D., Carrasco M.J., Alishetty S., Paige M., Alameh M.G., Weissman D. Nanomaterial delivery systems for mRNA vaccines. Vaccines. 2021;9:65. doi: 10.3390/vaccines9010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Park K.S., Sun X., Aikins M.E., Moon J.J. Non-viral COVID-19 vaccine delivery systems. Adv Drug Deliv Rev. 2021;169:137–151. doi: 10.1016/j.addr.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fukushi M., Yamashita M., Miyoshi-Akiyama T., Kubo S., Yamamoto K., Kudo K. Laninamivir octanoate and artificial surfactant combination therapy significantly increases survival of mice infected with Lethal influenza H1N1 virus. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bollag W.B., Gonzales J.N. Phosphatidylglycerol and surfactant: a potential treatment for COVID-19? Med Hypotheses. 2020;144:110277. doi: 10.1016/j.mehy.2020.110277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Heard D.D., Ashworth R.W. The colloidal properties of chlorhexidine and its interaction with some macromolecules. J Pharm Pharmacol. 1968;20:505–512. doi: 10.1111/j.2042-7158.1968.tb09798.x. [DOI] [PubMed] [Google Scholar]
- 152.Komljenović I., Marquardt D., Harroun T.A., Sternin E. Location of chlorhexidine in DMPC model membranes: a neutron diffraction study. Chem Phys Lipids. 2010;163:480–487. doi: 10.1016/j.chemphyslip.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 153.Lourith N., Kanlayavattanakul M. Natural surfactants used in cosmetics: Glycolipids. Int J Cosmet Sci. 2009;31:255–261. doi: 10.1111/j.1468-2494.2009.00493.x. [DOI] [PubMed] [Google Scholar]
- 154.Adu S.A., Naughton P.J., Marchant R., Banat I.M. Microbial biosurfactants in cosmetic and personal skincare pharmaceutical formulations. Pharmaceutics. 2020;12:1–21. doi: 10.3390/pharmaceutics12111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yuan L., Zhang S., Peng J., Li Y., Yang Q. Synthetic surfactin analogues have improved anti-PEDV properties. PLoS One. 2019;14 doi: 10.1371/journal.pone.0215227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Malavia N.K., Zurakowski D., Schroeder A., Princiotto A.M., Laury A.R., Barash H.E., et al. Liposomes for HIV prophylaxis. Biomaterials. 2011;32:8663–8668. doi: 10.1016/j.biomaterials.2011.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Law S.L., Huang K.J., Chiang C.H. Acyclovir-containing liposomes for potential ocular delivery Corneal penetration and absorption. J Contr Release. 2000;63:135–140. doi: 10.1016/S0168-3659(99)00192-3. [DOI] [PubMed] [Google Scholar]
- 158.Tahara K., Kobayashi M., Yoshida S., Onodera R., Inoue N., Takeuchi H. Effects of cationic liposomes with stearylamine against virus infection. Int J Pharm. 2018;543:311–317. doi: 10.1016/j.ijpharm.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 159.Maitani Y., Ishigaki K., Nakazawa Y., Aragane D., Akimoto T., Iwamizu M., et al. Polyethylenimine combined with liposomes and with decreased numbers of primary amine residues strongly enhanced therapeutic antiviral efficiency against herpes simplex virus type 2 in a mouse model. J Contr Release. 2013;166:139–146. doi: 10.1016/j.jconrel.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 160.Midoux P., Pichon C. Lipid-based mRNA vaccine delivery systems. Expert Rev Vaccines. 2015;14:221–234. doi: 10.1586/14760584.2015.986104. [DOI] [PubMed] [Google Scholar]
- 161.Arteta M.Y., Kjellman T., Bartesaghi S., Wallin S., Wu X., Kvist A.J., et al. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc Natl Acad Sci USA. 2018;115:E3351–E3360. doi: 10.1073/pnas.1720542115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Davies N., Hovdal D., Edmunds N., Nordberg P., Dahlén A., Dabkowska A., et al. Functionalized lipid nanoparticles for subcutaneous administration of mRNA to achieve systemic exposures of a therapeutic protein. Mol Ther - Nucleic Acids. 2021;24:369–384. doi: 10.1016/j.omtn.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Veldhuizen R.A.W., Zuo Y.Y., Petersen N.O., Lewis J.F., Possmayer F. The COVID-19 pandemic: a target for surfactant therapy? Expet Rev Respir Med. 2021;15:597–608. doi: 10.1080/17476348.2021.1865809. [DOI] [PubMed] [Google Scholar]
- 164.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 165.O'Donnell V.B., Thomas D., Stanton R., Maillard J.-Y., Murphy R.C., Jones S.A., et al. Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection. Function. 2020;1:zqaa002. doi: 10.1093/function/zqaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Meister T.L., Brüggemann Y., Todt D., Conzelmann C., Müller J.A., Groß R., et al. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2. J Infect Dis. 2020;222:1289–1292. doi: 10.1093/infdis/jiaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dennison D.K., Meredith G.M., Shillitoe E.J., Caffesse R.G. The antiviral spectrum of Listerine antiseptic. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:442–448. doi: 10.1016/S1079-2104(05)80124-6. [DOI] [PubMed] [Google Scholar]
- 168.Higgins T.S., Wu A.W., Illing E.A., Sokoloski K.J., Weaver B.A., Anthony BeP., et al. Intranasal antiviral drug delivery and coronavirus disease 2019 (COVID-19): a state of the art review. Otolaryngol Head Neck Surg. 2020;163:682–694. doi: 10.1177/0194599820933170. [DOI] [PubMed] [Google Scholar]