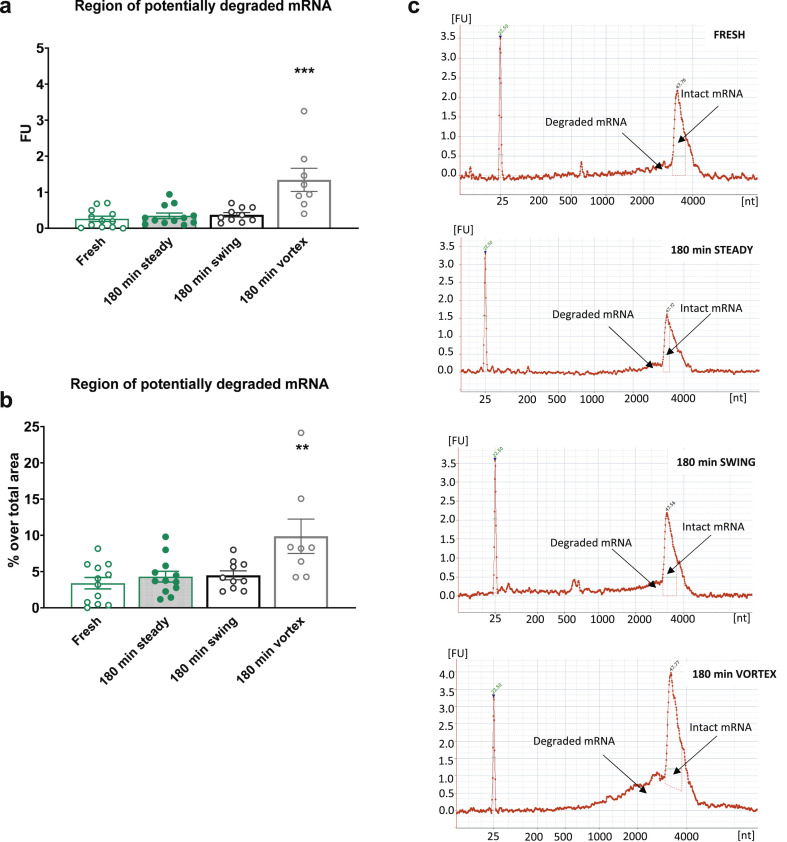
Fig. 1.
Analysis of the integrity of mRNA in reconstituted Pfizer–BioNTech coronavirus disease 2019 (COVID-19) vaccines. (a) Measurement of the region of potentially degraded mRNA in fluorescence units (FU) of the samples analysed immediately (fresh) or after 180 min without movement (180 min steady), with continuous swing shaker movement (180 min swing), or with Vortex shaking (180 min Vortex). Individual values of FU with the means ± SEMs are represented; one-way ANOVA, F (3,38) = 13.39, p < 0.001, Newman–Keus (N–K) post hoc test ∗∗∗p < 0.001 Vortex versus the remaining groups (n = 8–12). (b) Percentage over the region's total area of potentially degraded mRNA of the samples analysed immediately or after 180 min without movement, with continuous swing shaker movement, or with Vortex shaking. Individual values of FU percentages with the means ± SEMs are represented; one-way ANOVA, F (3,38) = 5.772, p < 0.01, N–K post hoc test ∗∗p < 0.01 Vortex versus the remaining groups (n = 8–12). (c) Representative electropherograms expressed in FU of RNA integrity for different mRNA samples, detailing the regions that indicate potentially degraded and intact mRNA peaks in the samples analysed immediately, or after 180 min without movement, with continuous swing shaker movement, or with Vortex shaking.