FIGURE 3. Seropositivity rates and 95% confidence intervals of dengue neutralising antibodies (measured by microneutralization assay) against individual and multiple serotypes in the study (“current study”), and in seronegative adolescents in Latin America enrolled in a separate phase 3 efficacy study (“Phase 3 efficacy study”). Per protocol set data.
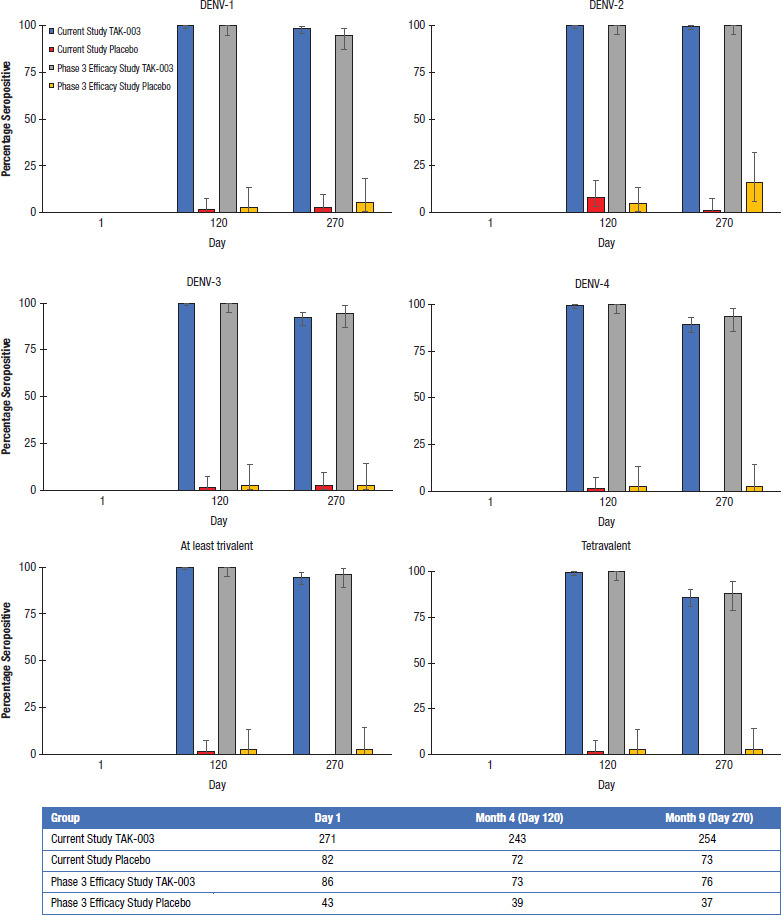
Number of participants with available data within protocol-specified visit window for each time point are given in the table below the figure
Immunogenicity data are presented for the per-protocol set (PPS), i.e. all participants who were seronegative at baseline, received at least one dose of TAK-003 or placebo, and had no major protocol deviations.
Seropositive was defined as a reciprocal neutralizing titer ≥10
Source: Figure prepared by the authors from current study and the phase 3 efficacy study data
