Abstract
Background
Pituitary apoplexy (PA) is a rare, but life-threatening, condition characterized by pituitary infarction and hemorrhage, most often in the setting of a preexisting adenoma. The risk factors and mechanisms associated with PA are poorly understood. Although neurovascular manifestations of coronavirus disease 2019 (COVID-19) infection have been documented, its association with PA has not yet been determined.
Methods
From a prospectively collected database of patients treated at a tertiary care center for pituitary adenoma, we conducted a retrospective medical record review of PA cases during the COVID-19 pandemic from March 2020 to December 2020. We also conducted a literature review to identify other reported cases.
Results
We identified 3 consecutive cases of PA and concomitant COVID-19 infection. The most common symptoms at presentation were headache and vision changes. The included patients were successfully treated with surgical decompression and medical management of the associated endocrinopathy, ultimately experiencing improvement in their visual symptoms at the latest follow-up examination. COVID-19 infection in the perioperative period was corroborated by polymerase chain reaction test results in all the patients.
Conclusions
With the addition of our series to the literature, 10 cases of PA in the setting of COVID-19 infection have been confirmed. The present series was limited in its ability to draw conclusions about the relationship between these 2 entities. However, COVID-19 infection might represent a risk factor for the development of PA. Further studies are required.
Key words: Coronavirus, COVID-19, Neurosurgery, Pituitary adenoma, Pituitary apoplexy
Abbreviations and Acronyms: ACE-2, Angiotensin-converting enzyme-2; COVID-19, Coronavirus disease 2019; MRI, Magnetic resonance imaging; PA, Pituitary apoplexy; PCR, Polymerase chain reaction
Introduction
As of May 2021, the coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected 192 countries and left >3 million people dead.1 Although primarily a respiratory disease, the coagulopathic, inflammatory, and neurologic manifestations of COVID-19 infection have been documented and represent potential therapeutic targets to limit the morbidity and mortality.2, 3, 4, 5 Neurologic sequelae, even among those without severe pulmonary infection, include impaired consciousness and cognition, seizure, neurovascular ischemia, and intracranial hemorrhage.6, 7, 8
Pituitary apoplexy (PA) is a life-threatening condition characterized by intraparenchymal hypophyseal infarction and/or hemorrhage, often in the setting of preexisting pituitary adenoma.9 It is relatively rare in the general population, with symptomatic cases occurring in ∼10% of those with pituitary adenoma.10 Patients will typically present with sudden headache, vision changes, and endocrinopathy.11 PA in association with an underlying COVID-19 diagnosis has been reported; however, the presence of confounding variables (i.e., concomitant anticoagulation therapy and/or the predilection of COVID-19 for critically ill patients) has limited the ability to draw conclusions of causation.12 Nevertheless, the propensity for COVID-19 to propagate microvascular ischemia, such as occurs with PA, has been characterized and represents a logical connection between the 2 entities.5 , 8 , 13, 14, 15 In the present report, we have described 3 consecutive patients with PA, minimal comorbidity, and concurrent COVID-19 infection in the perioperative period who had been treated at our tertiary care center. In the context of a literature review, we have discussed the potential clinical and pathophysiologic relationship between these 2 conditions.
Methods
We performed a retrospective case series of patients who had been admitted to a single tertiary care hospital for neurosurgical management of PA from March 2020 to December 2020. Special attention was given toward the temporal relationship between the symptom onset of PA and the COVID-19 diagnosis during or shortly before admission. The medical records and imaging examinations were retrospectively reviewed. These data were presented to the institutional review board of our institution, which waived the need for patient informed consent owing to the anonymized retrospective case-series design.
To strengthen the discussion of a potential relationship between PA and COVID-19, we also conducted a literature review. A search of the PubMed and Google Scholar databases was performed on May 11, 2021. The search terms included combinations of 1) “pituitary apoplexy,” “pituitary hemorrhage,” and “hypopituitarism”; and 2) “COVID-19,” “coronavirus,” and “SARS-CoV-2.” Case reports and/or studies that had reported on the relationship between COVID-19 and PA or the occurrence of abrupt hypopituitarism were of particular interest.
Results
Three patients (2 women and 1 man; average age, 54 ± 2 years) had been treated for PA during the 10-month study period. All 3 patients had also been diagnosed with confirmed COVID-19 during or shortly before their admission, with no patient with PA presenting to our institution without concomitant COVID-19. These patients did not have any intracranial lesions other than PA, had not experienced any additional obvious neurologic complications from the COVID-19 infection, and had not been receiving anticoagulation therapy before their PA diagnosis. All relevant laboratory values, such as D-dimer, fibrinogen, platelet count, prothrombin time (PT), and activated partial thromboplastin time (aPTT), were within the normal limits for all 3 patients. Two of the patients had a medical history of hypertension and obesity. However, no pertinent history for a systemic autoimmune disease (i.e., endotheliitis or vasculitis) was described for any of the 3 patients. All 3 patients had presented with vision dysfunction and headache, in addition to the respiratory symptoms of their viral pneumonia. Using the 2007 American Thoracic Society guidelines for community-acquired pneumonia, all 3 patients met the criteria for nonsevere pulmonary disease.16 The cases of our patients are described in the next sections.
Patient 1
A 54-year-old woman was transferred to our center from an outside hospital with the complaint of a holocranial headache that had started 1 week previously. The patient reported that 2 days earlier, the headache had acutely worsened, with a new focal retro-orbital component. She also noted blurry vision in her right eye 1 day later but denied frank diplopia. On neurologic examination, she was awake and alert, and the cranial nerve findings were unremarkable. Her visual acuity was subjectively normal on the left. However, she could only perceive light on the right. Magnetic resonance imaging (MRI) demonstrated a hemorrhagic mass in the region of an enlarged sella turcica, along with suprasellar extension, suggesting a previously undetected tumor. PA was suspected within the parenchyma of a preexisting adenoma.
During her workup, the patient had endorsed contact with her grandson ∼1 week earlier coincidental with her headache onset. Her grandson was subsequently confirmed to be positive for COVID-19 infection. Because of this history and her symptoms, strict COVID-19 precautions and isolation measures were started for the patient. Subsequent polymerase chain reaction (PCR) test results were positive for COVID-19. The patient underwent resection of her sellar mass on the day of presentation via right frontoparietal craniotomy without complications.
Her headache had resolved shortly after the procedure. At the 1-month follow-up, her right visual acuity dysfunction had significantly improved, from only light perception preoperatively to 20/50 and right hemianopsia. Postoperatively, the patient required substitutive treatment with hydrocortisone, levothyroxine, and desmopressin. Pathologic examination confirmed a hemorrhagic null cell adenoma. The imaging studies are presented in Figure 1 .
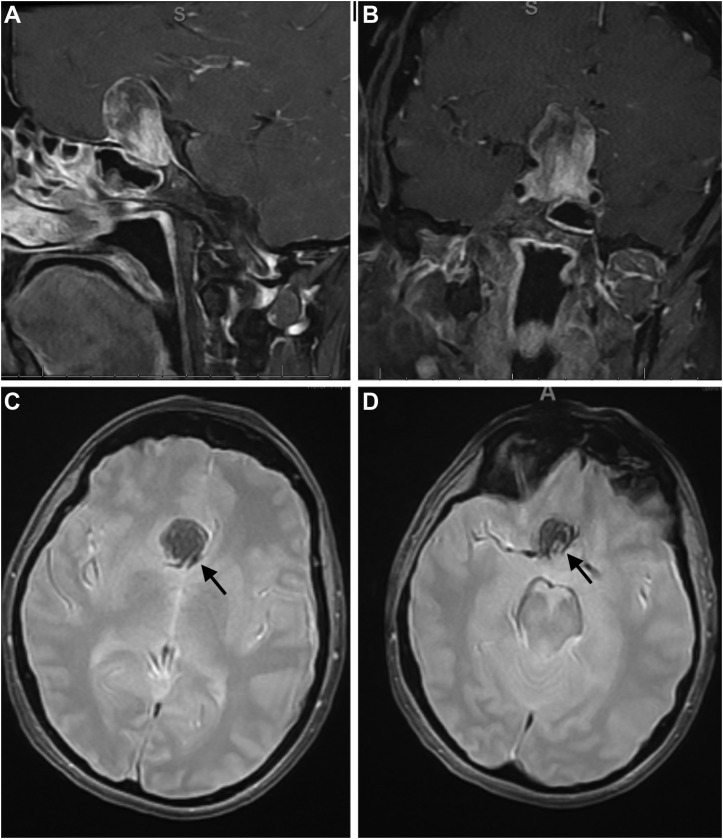
Figure 1.
Imaging studies for patient 1. Preoperative (A) sagittal and (B) coronal T1-weighted contrast-enhanced magnetic resonance imaging scans demonstrating a 2.8-cm mixed hyperintense-hypointense sellar lesion, compatible with a pituitary adenoma with blood products at different stages of degradation. (C and D) Preoperative axial gradient echo sequencing images confirming accentuated flow signal in the sellar lesion (black arrows).
Patient 2
A 56-year-old obese man with a history of hypertension and hypothyroidism had been transferred from an outside hospital with the complaint of a 1-week history of headache. Initially, he was prescribed analgesia, received fluids, and was discharged home. However, owing to his headache's persistence and the new onset of binocular diplopia, he presented to his primary care physician. At that time, computed tomography of the head revealed a sellar hemorrhagic mass, and he was subsequently transferred to our tertiary care center.
On arrival, the neurologic examination revealed altered mental status and complete third and fourth cranial nerve palsies. No visual deficits were objectively assessed. However, given his altered mental status, MRI was promptly ordered, which confirmed a sellar hemorrhagic lesion with invasion of the right cavernous sinus. After initial stabilization, further questioning revealed that the patient had begun experiencing chills and myalgias ∼10 days before admission. No confirmed community exposure was identified; however, PCR testing demonstrated positivity for COVID-19 infection. Precautions were taken, and the patient was isolated in accordance with our institutional protocol. He subsequently underwent endonasal transsphenoidal microscopic resection of the sellar mass on the day of admission without complications.
The patient's postoperative course was uneventful, and he was discharged home on the second postoperative day with no evidence of hyponatremia or diabetes insipidus, although he did require hydrocortisone and levothyroxine supplementation. At the 6-week follow-up visit, the neurologic examination revealed complete resolution of his third and fourth cranial nerve palsies. The final pathologic examination demonstrated necrotic and hemorrhagic tissue within a lactotroph-type pituitary adenoma, confirming the diagnosis of PA. The imaging studies are presented in Figure 2 .
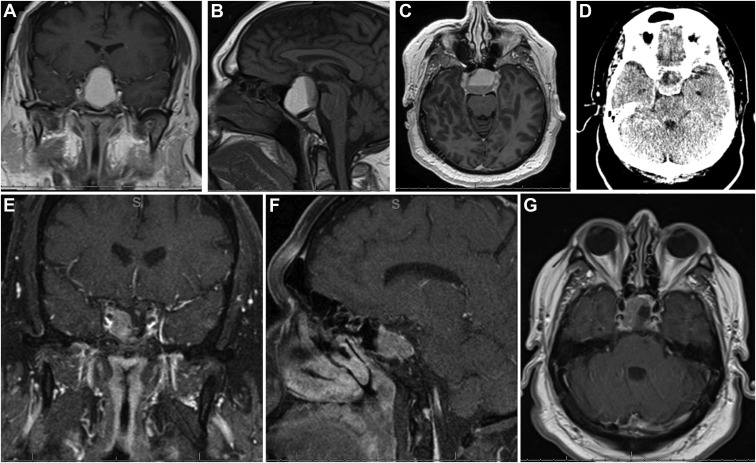
Figure 2.
Imaging studies for patient 2. Preoperative (A) coronal T1-weighted, precontrast, (B) coronal T1-weighted contrast-enhanced, and (C) sagittal T1-weighted contrast-enhanced magnetic resonance imaging scans demonstrating pituitary apoplexy in a 1.8-cm macroadenoma with interval enlargement. (D and E) Preoperative axial susceptibility-weighted imaging scans demonstrating signal in the adenoma consistent with hemorrhage (white arrows). Postoperative (F) coronal T1-weighted contrast-enhanced, (G) sagittal T1-weighted contrast-enhanced, and (H) axial T1-weighted contrast-enhanced magnetic resonance imaging scans demonstrating adequate decompression of the optic apparatus.
Patient 3
A 52-year-old obese man with a history of hypertension had presented to the emergency department complaining of acute headache. The patient endorsed that during the previous year, he had been experiencing progressive peripheral vision loss that had started in his left eye and had begun to involve the right eye after a few months. He also reported decreased libido and impotence for ∼2 years before his presentation. The patient was then evaluated by ophthalmology, who confirmed his bitemporal hemianopsia. These findings prompted MRI with a pituitary protocol, which revealed a sellar mass with a significant hemorrhagic component, sellar remodeling, and upward displacement of the optic chiasm. At follow-up 3 days later, the patient reported his headaches were increasing in intensity with no improvement in his visual symptoms. He subsequently was scheduled for elective endoscopic transsphenoidal resection.
The patient's preoperative pituitary laboratory test results demonstrated central hypothyroidism, hypogonadism, low insulin-like growth factor-1, and low-to-normal cortisol levels. His surgery was uneventful. Intraoperatively, a predominantly liquefied hemorrhagic mass was identified with necrotic tissue, especially along the posterior aspect of a markedly expanded sella. One day after surgery, the patient began to experience a cough and shortness of breath, accompanied by low-grade fever and chills. PCR testing for COVID-19 infection was positive.
After surgery, hydrocortisone therapy was begun for hypocortisolism, and the patient was discharged home on postoperative day 3 with no evidence of hyponatremia or diabetes insipidus. Postoperatively, the patient made an excellent recovery with complete reversal of his visual disturbances. Pathologic examination confirmed a lactotroph pituitary adenoma. The imaging studies are presented in Figure 3 .
Figure 3.
Imaging studies for patient 3. Preoperative (A) coronal T1-weighted contrast-enhanced, (B) sagittal T1-weighted contrast-enhanced, and (C) axial T1-weighted contrast-enhanced magnetic resonance imaging scans and (D) axial computed tomography scan demonstrating a sellar lesion with suprasellar extension and a fluid level compatible with intratumoral subacute bleeding. Postoperative (E) coronal T1-weighted contrast-enhanced, (F) sagittal T1-weighted contrast-enhanced, and (G) axial T1-weighted contrast-enhanced magnetic resonance imaging scans demonstrating decompression of the optic chiasm with residual tumor extending into the right cavernous sinus.
Literature Review
We identified 9 cases of pituitary dysfunction within the setting of suspected SARS-CoV-2 infection reported since March 2020.17, 18, 19, 20, 21, 22, 23, 24, 25 One pediatric patient, a 9-year-old girl had developed a suprasellar nongerminomatous germ cell tumor and asymptomatic COVID-19.23 Another patient, a 28-year-old woman, had been confirmed to have PA, although it was not clear whether the patient had ever contracted COVID-19.24 She had demonstrated spontaneous resolution of her PA after a surgical delay.24 Although of interest and relevance to our study, these 2 cases were subsequently excluded. The cases of the 7 remaining patients are presented with the cases of our 3 patients in Table 1 .
Table 1.
Literature Review of 10 Reported Cases of PA in the Setting of Confirmed COVID-19 Infection
| Investigator | Patient | Notable Comorbidities | PA Presentation | COVID-19 Respiratory Severity26 | MRI Findings | Outcome |
|---|---|---|---|---|---|---|
| Bordes et al.19 | 65-Year-old woman | Hypertension | Frontal headache, phonophobia, photophobia | Not severe | 1.4-cm Heterogeneous component without identifiable adenoma | Corticosteroid therapy and discharge |
| Solorio-Pineda et al.17 | 27-Year-old man | Unremarkable | Frontal headache, altered mental status, decreased visual acuity | Severe | 5.9 × 5.2 × 6.8-cm Heterogeneous sellar mass | Died of pulmonary complications; surgical intervention of PA not initiated |
| Ghosh et al.21 | 44-Year-old woman | Unremarkable | Severe headache, diplopia | Not severe | 2.4 × 2.5 × 3.1-cm Heterogeneous cystic sellar mass with fluid–fluid levels | Patient refused surgical intervention; discharge with slow symptom improvement at follow-up |
| Chan et al.18 | 28-Year-old woman | Pregnant in third trimester | Mild headache, vision loss in left eye | Not severe | 2.2 × 2.5 × 2.0-cm Cystic and hemorrhagic sellar mass with enlarged sella | TSS after delivery; discharge with complete recovery |
| dos Santos e Santos et al.25 | 47-Year-old man | Unremarkable | Frontal headache, diplopia, vision loss in left eye | Not severe | 1.9 × 2.8 × 2.0-cm Hyperdense sellar mass with optic chiasm impingement | TSS; discharge with complete recovery |
| Katti et al.22 | 46-Year-old man | Unremarkable | Headache, acute bilateral vision loss | Not severe | 3.4 × 3 × 2.4-cm Heterogeneous sellar/suprasellar mass with optic chiasm impingement | Corticosteroid therapy and discharge |
| LaRoy et al.20 | 35-Year-old man | Unremarkable | Severe retro-orbital headache, neck stiffness | Not severe | 0.7 × 0.8 × 0.8-cm Small hyperdense blood collection within sella turcica | Discharge |
| Present study | 54-Year-old woman | Unremarkable | Holocranial headache, blurry vision | Not severe | 2.8-cm Heterogeneous sellar mass | Transcranial resection; discharge |
| Present study | 56-Year-old man | Obesity, hypertension, hypothyroidism | Headache, diplopia | Not severe | 1.8-cm Sellar mass with interval enlargement and acute hemorrhage | TSS; discharge |
| Present study | 52-Year-old man | Obesity, hypertension | Peripheral vision loss, impotence | Not severe | Sellar lesion with suprasellar extension and T1-weighted hyperintense fluid level | TSS; discharge |
COVID-19, coronavirus disease 2019; MRI, magnetic resonance imaging; PA, pituitary apoplexy; TSS, transsphenoidal surgery.
Discussion
PA is a clinical emergency. Although neurologic manifestations of COVID-19 have been reported,2 , 3 the relationship between COVID-19 and PA has yet to be established. We have reported 3 confirmed cases of PA and concomitant COVID-19 infection, providing suggestive evidence of an association between the 2 entities.
Epidemiology and Pathophysiology
PA is rare, with a prevalence of 6.2 per 100,000 persons, with symptomatic PA occurring in ∼2%–12% of patients with adenoma.27 Most patients affected range in age from 37 to 58 years, and men are nearly twice as likely to present with PA than are women.26 It is generally accepted that PA is a consequence of spontaneous intrasellar hemorrhage and/or infarction of the hypophysis, typically in the setting of a preexisting adenoma.11 Although its exact mechanism is not fully understood, several predisposing factors have been suggested. In a 2015 review of 1202 cases, Briet et al.10 reported that angiography, surgery (i.e., cardiac, orthopedic, other), closed head trauma, dynamic testing (i.e., dexamethasone suppression), suprasellar macroadenoma extension, pregnancy, chronic hypertension, increased intracranial pressure, and therapy with dopamine or gonadotropin-releasing hormone agonists have all been proposed. Common etiologic themes leading to PA include acute derangements in blood pressure (especially hypotension), anticoagulation therapy, and greater metabolic demands in the setting of chronically stressed hypophyseal vasculature.10 In contrast to the hypophyseal portal system of the normal pituitary gland, pituitary adenomas derive most of their blood supply from direct arterial branches.10 As the sellar mass grows, the vascular reservoir for the adenoma decreases amidst a reduction in angiogenesis.28 , 29 Furthermore, the normal endothelial organization of the capillary bed has been observed to be replaced by an arteriolar arrangement in the setting of an adenoma, limiting erythrocyte migration into the tumor.30 Postmortem histologic brain examinations of patients with COVID-19 revealed a propensity for hypoxic brain damage and neuroinflammation.31 , 32 These 2 processes together might explain endothelial dysfunction in the hypophyseal vasculature in the occurrence of PA associated with COVID-19 infection.
Previous studies have described a syndrome of hypopituitarism in the presence of flavivirus or bunyavirus infection.33 , 34 This appears to result from complications of viral tropism for the pituitary gland and ischemic and/or hemorrhagic sequelae from vascular damage. A similar pathophysiologic mechanism has been proposed for PA in the setting of COVID-19 infection amid accumulating evidence suggesting that COVID-19 targets the nervous system.12 The reported rates of stroke in patients with COVID-19 infection have ranged from 0.9% to 5.7%,35 , 36 and the incidence of venous thromboembolism has been as high as 36% in patients admitted to the intensive care unit.37 In 1 study of 32 critically ill patients with COVID-19, 8 (25%) had experienced severe central nervous system involvement.38 Similarly, intracranial hemorrhage or cerebral microbleeding events have been reported in ≤22% of critically ill patients.38 Patients with COVID-19 infection who experience stroke are more likely to be younger and to have a higher National Institutes of Health Stroke Scale score at admission.36 COVID-19 has shown affinity for the angiotensin-converting enzyme-2 (ACE-2) receptor on the host cells of the central nervous system and vasculature.12 , 39 This leads to cellular internalization of the virus,39 where COVID-19 downregulates ACE-2 receptors and leads to oxidative stress, vasodilation, neuroinflammation, and thrombogenesis. Vascular dysautoregulation and microischemia subsequently ensue.8 , 11
With the addition of our series, we found 10 cases of PA concurrent with COVID-19 infection reported in the literature. In all but 1 of these patients, a preexisting macroadenoma was identified. Bordes et al19 reported sellar hemorrhage without an underlying tumor, which could be secondary to the relatively older age of the patient and their comorbid hypertension. Hypertension was similarly seen in 2 of our patients, representing a potential risk factor for PA in the setting of acute respiratory infection, regardless of macroadenoma presence or size.10 , 12 Although the increased risk of coagulopathy associated with COVID-19 infection is related to elevated fibrinogen and D-dimer levels, mild thrombocytopenia, and normal or mild prolongation of PT and aPTT,40 the laboratory values for all 3 of our patients were within normal limits. This might prompt the initiation of unfractionated or low-molecular-weight heparin, representing another potential risk factor for PA. However, none of our patients had received anticoagulation therapy; thus, PA could represent the natural history of a predisposed pituitary adenoma patient susceptible to COVID-19 viral tropism, thrombocytopenia, coagulopathy, and abrupt blood pressure changes even without concerning laboratory values12 (Figure 4 ).
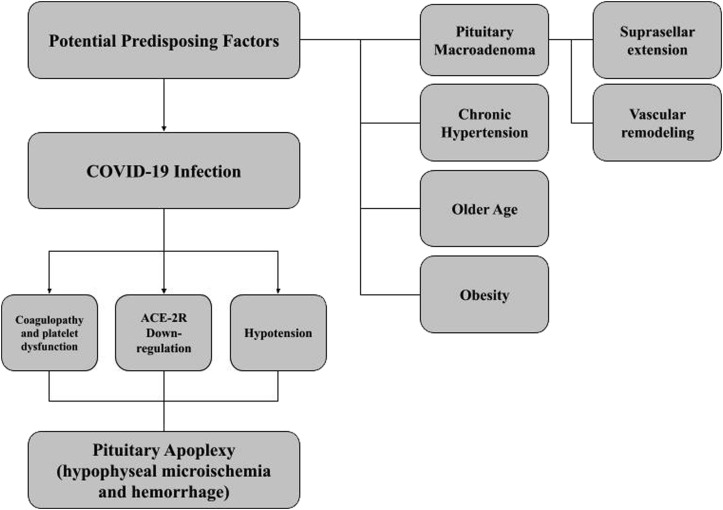
Figure 4.
Proposed pathophysiologic mechanism underlying coronavirus disease 2019 (COVID-19) propagation of pituitary apoplexy in patients with underlying adenoma. ACE-2R, angiotensin-converting enzyme-2 receptor.
In accordance with the Bradford-Hill criteria, which assess the epidemiological evidence of causality between 2 phenomena,41 our findings strengthen the association between PA and COVID-19, given that ours is the largest consecutive series to date. Additionally, the consistency of our results (all patients treated at 1 neurosurgery department) and the absence of clear predisposing factors, other than hypertension and obesity in 2 patients (specificity), might improve confidence in our evidence. The other risk factors for PA, including head trauma, intracranial hypertension, radiotherapy, pregnancy, and anticoagulation,10 , 11 , 42 were not present in any of our 3 patients. Temporality is also important to establish causality and was met in our report, especially for patients 1 and 2. These 2 patients had had a positive COVID-19 test within 1 month before their admission for PA. Patient 3 had tested positive for COVID-19 at 4 days after the diagnosis of PA, which warrants extra caution in concluding a causal relationship. In contrast, however, considering that the mean incubation time for COVID-19 time is 5 days,43 this patient could have already been infected and asymptomatic when he experienced symptoms relative to the pituitary infarction. Additionally, 9 of the reported patients with PA had had nonsevere pulmonary infections, which could also support the idea that PA can manifest regardless of COVID-19 severity or symptoms. This relationship also requires confirmation.
Management and Outcomes
Patients who present with symptoms of PA and suspected COVID-19 infection or in areas of high community spread should undergo nasopharyngeal reverse transcription PCR and be isolated in accordance with institutional and public health authority standards. Evidence has demonstrated the presence of SARS-CoV-2 in the cerebrospinal fluid, which might raise suspicion about the viral relationship and bleeding diathesis.44 Reverse transcription PCR testing of the cerebrospinal fluid could illuminate the presence of direct invasion of the virus into the central nervous system and should be considered in the routine care of patients with PA and confirmed COVID-19 infection. If immediate surgery is required, pituitary management protocols should be initiated to protect the treating staff and limit viral transmission. For nonemergent PA, patients should be treated conservatively where possible and monitored closely and undergo surgery when their COVID-19 infection has resolved.
From a PA management perspective, it is worthwhile to highlight the importance of monitoring the fluid and electrolyte balance and correcting pituitary hormone deficiencies.11 Of particular interest is weighing the benefits and risk of anticoagulation and antiplatelet therapy when concerned for thrombosis in patients with pituitary macroadenoma and COVID-19, especially in the presence of laboratory evidence of derangements in D-dimer, fibrinogen, platelets, and PT and aPTT.40 Preemptive surgery might be warranted in these cases. Ibuprofen has also been associated with an increased number of ACE-2 receptors within the cell membrane, which might increase the infectivity of SARS-CoV-2.45 Thus, clinicians should be wary of administering ibuprofen to patients with COVID-19 and patients with known macroadenoma until COVID-19 has been ruled out, considering that headache is a common presenting symptom of PA. Once the patient has been stabilized, the decision to perform surgery depends heavily on the patient's visual status. PA is generally considered a surgical emergency when associated with acute visual deterioration.9 , 11 However, some patients have experienced spontaneous visual improvement with conservative corticosteroid therapy, fluid resuscitation, and hormonal supplementation.12 , 46 , 47 We attempted conservative management for 1 of our patients (patient 2), given the subacute progression of his visual symptoms. However, his vision did not improve after a short interval of observation, which prompted surgical resection.
Surgery can be performed via a transsphenoidal or transcranial approach, which should be determined by the morbidity of the individual patient and how it relates to COVID-19 infection.9 , 11 , 48 A theoretically increased risk exists of intraoperative COVID-19 transmission during endoscopic transsphenoidal surgery secondary to viral particle aerosolization, and the risk should be considered in the center-dependent protocols to protect healthcare staff. In June 2020, Fleseriu et al.49 and the Professional Education Committee of the Pituitary Society for Pituitary Surgery recommended systematically triaging pituitary patients for the severity of presentation, querying for COVID-19 symptoms, screening for COVID-19 infection via nasopharyngeal swab and chest radiography, and isolation for 2 weeks before surgery. In emergent cases such as PA, which cannot be deferred, full personal protective equipment for all treating clinicians should be worn and the use of a transcranial approach considered. Ultimately, however, little evidence is available that iatrogenic nasopharyngeal aerosolization of SARS-CoV-2 occurs50; thus, treating physicians should continue to follow location-specific government- and institution-dependent protocols for surgical management of pituitary pathology. For our series, the transcranial route was chosen for patient 1, primarily to rapidly decompress the third cranial nerve and address the tumor's lateral expansion. The selection of the approach for all our patients was ultimately determined primarily by surgeon preference, in line with our hospital's protocol.
Ultimately, clinical morbidity and mortality should represent the outcomes of interest in these patients, whether due to sequelae of their PA or other COVID-19–related manifestations.12 Although the short-term outcomes, such as endocrinopathy, ophthalmoplegia, respiratory dysfunction, and subsequent hospital readmission, are important for these patients and have been noted in the few cases reported thus far, the long-term prognosis remains to be elucidated. These concerns, along with system-based outcomes, represent potential inflection points for clinical decision-making among pituitary surgery teams.
Study Limitations
Our study had some limitations. Most importantly, its descriptive nature and the small sample size prevented definitive conclusions regarding the true relationship between PA and COVID-19, especially temporally with patient 3. We were similarly unable to compare our 3 patients with a control group owing to unavailable data, which also prohibited a statistical analysis. We also could not take sequences, such as gradient echo sequences, except for in patient 1, during the prospective MRI studies, which would have been useful. The 3 cases were diagnosed at a single center, which could have resulted in historical, confirmation, and design biases.
Future Perspectives
Although the relationship between PA and COVID-19 remains controversial, we believe that our study has provided necessary and relevant preliminary data. With the pandemic still significantly affecting multiple countries, multi-institutional clinical studies might improve the neurosurgical and critical care treatment of these patients. In the long term, the pathogenesis of PA remains to be fully determined. Given the microvascular manifestations of COVID-19, this might represent an opportunity to conduct translational work postmortem and in animal models to determine how patients with pituitary adenoma progress to infarction and/or hemorrhage. Regarding the radiographic diagnosis, when patients have been confirmed to have COVID-19 before their admission for PA, we recommend MRI with a pituitary protocol and gradient echo sequences, which can detect even very minor differences in signal homogeneity.43 This could more effectively guide COVID-19 and PA management and strengthen the confidence of an association between these 2 pathologies.
Conclusions
With the addition of our 3-patient case series, 10 cases of PA in the setting of confirmed COVID-19 infection have been reported. These cases suggest a relationship between these 2 highly morbid conditions. Molecular downregulation of cellular ACE-2 receptors, vascular dysautoregulation, abrupt hypotension, and coagulopathy could, together, propagate pituitary infarction and hemorrhage, with the presence of a pituitary macroadenoma representing a likely risk factor. Comparative studies are still required to connect these two conditions on a pathophysiologic basis. However, until then, COVID-19 should be considered a possible risk factor for PA and management adjusted accordingly.
CRediT authorship contribution statement
Rafael Martinez-Perez: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Visualization, Writing - original draft. Michael W. Kortz: Project administration, Visualization, Writing - review & editing. Benjamin W. Carroll: Resources. Daniel Duran: Resources. James S. Neill: Resources. Gustavo D. Luzardo: Funding acquisition. Marcus A. Zachariah: Funding acquisition, Investigation, Methodology, Supervision, Validation, Writing - review & editing.
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Johns Hopkins University of Medicine Coronavirus Resource Center. https://coronavirus.jhu.edu/ Available at:
- 2.Carod Artal F.J. Complicaciones neurológicas por coronavirus y COVID-19. Rev Neurol. 2020;70:311. doi: 10.33588/rn.7009.2020179. [DOI] [PubMed] [Google Scholar]
- 3.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helms J., Kremer S., Merdji H., et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan N.C., Weitz J.I. COVID-19 coagulopathy, thrombosis, and bleeding. Blood. 2020;136:381–383. doi: 10.1182/blood.2020007335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dogra S., Jain R., Cao M., et al. Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. 2020;29:104984. doi: 10.1016/j.jstrokecerebrovasdis.2020.104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain R., Young M., Dogra S., et al. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414:116923. doi: 10.1016/j.jns.2020.116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vattoth S., Abdelhady M., Alsoub H., Own A., Elsotouhy A. Critical illness-associated cerebral microbleeds in COVID-19. Neuroradiol J. 2020;33:374–376. doi: 10.1177/1971400920939229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barkhoudarian G., Kelly D.F. Pituitary apoplexy. Neurosurg Clin N Am. 2019;30:457–463. doi: 10.1016/j.nec.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Briet C., Salenave S., Bonneville J.-F., Laws E.R., Chanson P. Pituitary apoplexy. Endocr Rev. 2015;36:622–645. doi: 10.1210/er.2015-1042. [DOI] [PubMed] [Google Scholar]
- 11.Turgut M., Özsunar Y., Başak S., Güney E., Kır E., Meteoğlu İ. Pituitary apoplexy: an overview of 186 cases published during the last century. Acta Neurochir. 2010;152:749–761. doi: 10.1007/s00701-009-0595-8. [DOI] [PubMed] [Google Scholar]
- 12.Frara S., Allora A., Castellino L., di Filippo L., Loli P., Giustina A. COVID-19 and the pituitary. Pituitary. 2021;24:465–481. doi: 10.1007/s11102-021-01148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melmed K.R., Cao M., Dogra S., et al. Risk factors for intracerebral hemorrhage in patients with COVID-19. J Thromb Thrombolysis. 2021;51:953–960. doi: 10.1007/s11239-020-02288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharifi-Razavi A., Karimi N., Rouhani N. COVID-19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect. 2020;35:100669. doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegler J.E., Cardona P., Arenillas J.F., et al. Cerebrovascular events and outcomes in hospitalized patients with COVID-19: the SVIN COVID-19 multinational registry. Int J Stroke. 2021;16:437–447. doi: 10.1177/1747493020959216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metlay J.P., Waterer G.W., Long A.C., et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solorio-Pineda S., Almendárez-Sánchez C.A., Tafur-Grandett A.A., et al. Pituitary macroadenoma apoplexy in a severe acute respiratory syndrome-coronavirus-2-positive testing: causal or casual? Surg Neurol Int. 2020;11:304. doi: 10.25259/SNI_305_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan J.L., Gregory K.D., Smithson S.S., Naqvi M., Mamelak A.N. Pituitary apoplexy associated with acute COVID-19 infection and pregnancy. Pituitary. 2020;23:716–720. doi: 10.1007/s11102-020-01080-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bordes S.J., Phang-Lyn S., Najera E., Borghei-Razavi H., Adada B. Pituitary apoplexy attributed to COVID-19 infection in the absence of an underlying macroadenoma or other identifiable cause. Cureus. 2021;13:e13315. doi: 10.7759/cureus.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaRoy M., McGuire M. Pituitary apoplexy in the setting of COVID-19 infection: a case report. https://doi.org/10.1016/j.ajem.2021.02.045 [e-pub ahead of print]. Am J Emerg Med. [DOI] [PMC free article] [PubMed]
- 21.Ghosh R., Roy D., Roy D., et al. A rare case of SARS-CoV-2 infection associated with pituitary apoplexy without comorbidities. J Endocr Soc. 2021;5:bvaa203. doi: 10.1210/jendso/bvaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katti V., Ramamurthy L.B., Kanakpur S., Shet S.D., Dhoot M. Neuro-ophthalmic presentation of COVID-19 disease: a case report. Indian J Ophthalmol. 2021;69:992–994. doi: 10.4103/ijo.IJO_3321_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaudino R., Orlandi V., Cavarzere P., et al. Case report: SARS-CoV-2 infection in a child with suprasellar tumor and hypothalamic-pituitary failure. Front Endocrinol (Lausanne) 2021;12:596654. doi: 10.3389/fendo.2021.596654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bray D.P., Solares C.A., Oyesiku N.M. Rare case of a disappearing pituitary adenoma during the coronavirus disease 2019 (COVID-19) pandemic. World Neurosurg. 2021;146:148–149. doi: 10.1016/j.wneu.2020.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.dos Santos e Santos C., da Costa Lima L.M., Santos C.A.T., Neill J.S., Vale H.F., Kurnutala L.N. Pituitary tumor resection in a patient with SARS-CoV-2 (COVID-19) infection: a case report and suggested airway management guidelines. Braz J Anesthesiol. 2020;70:165–170. doi: 10.1016/j.bjane.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricciuti R., Nocchi N., Arnaldi G., Polonara G., Luzi M. Pituitary adenoma apoplexy: review of personal series. Asian J Neurosurg. 2018;13:560–564. doi: 10.4103/ajns.AJNS_344_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez A., Karavitaki N., Wass J.A.H. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK) Clin Endocrinol (Oxf) 2010;72:377–382. doi: 10.1111/j.1365-2265.2009.03667.x. [DOI] [PubMed] [Google Scholar]
- 28.Oldfield E.H., Merrill M.J. Apoplexy of pituitary adenomas: the perfect storm. J Neurosurg. 2015;122:1444–1449. doi: 10.3171/2014.10.JNS141720. [DOI] [PubMed] [Google Scholar]
- 29.Turner H.E., Nagy Z., Gatter K.C., Esiri M.M., Harris A.L., Wass J.A. Angiogenesis in pituitary adenomas and the normal pituitary gland. J Clin Endocrinol Metab. 2000;85:1159–1162. doi: 10.1210/jcem.85.3.6485. [DOI] [PubMed] [Google Scholar]
- 30.Schechter J., Goldsmith P., Wilson C., Weiner R. Morphological evidence for the presence of arteries in human prolactinomas. J Clin Endocrinol Metab. 1988;67:713–719. doi: 10.1210/jcem-67-4-713. [DOI] [PubMed] [Google Scholar]
- 31.Solomon I.H., Normandin E., Bhafacharyya S., et al. Neuropathological features of COVID-19. N Engl J Med. 2020;383:989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matschke J., Lütgehetmann M., Hagel C., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhoelan S., Langerak T., Noack D., et al. Hypopituitarism after orthohantavirus infection: what is currently known? Viruses. 2019;11:340. doi: 10.3390/v11040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan S.K., Seow C.J., Tan E., Chau Y.P., Dalan R. Pituitary apoplexy secondary to thrombocytopenia due to dengue hemorrhagic fever: a case report and review of the literature. Endocr Pract. 2014;20:e58–e64. doi: 10.4158/EP13319.CR. [DOI] [PubMed] [Google Scholar]
- 35.Li Y., Li M., Wang M., et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5:279–284. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaghi S., Ishida K., Torres J., et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lodigiani C., Iapichino G., Carenzo L., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keller E., Brandi G., Winklhofer S., et al. Large and small cerebral vessel involvement in severe COVID-19: detailed clinical workup of a case series. Stroke. 2020;51:3719–3722. doi: 10.1161/STROKEAHA.120.031224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Divani A.A., Andalib S., Biller J., et al. Central nervous system manifestations associated with COVID-19. Curr Neurol Neurosci Rep. 2020;20:60. doi: 10.1007/s11910-020-01079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen E.C., Zon R., BaJnelli E., Connors J. Approach to the patient with COVID-19-associated thrombosis: a case-based review. Oncologist. 2020;25:e1500–e1508. doi: 10.1634/theoncologist.2020-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fedak K.M., Bernal A., Capshaw Z.A., Gross S. Applying the Bradford Hill criteria in the 21st century: how data integration has changed causal inference in molecular epidemiology. Emerg Themes Epidemiol. 2015;12:14. doi: 10.1186/s12982-015-0037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nawar R.N., AbdelMannan D., Selman W.R., Arafah B.M. Pituitary tumor apoplexy: a review. J Intensive Care Med. 2008;23:75–90. doi: 10.1177/0885066607312992. [DOI] [PubMed] [Google Scholar]
- 43.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 44.Moriguchi T., Harii N., Goto J., et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim S.H., Lee K.C., Kim S.H. Cranial nerve palsies accompanying pituitary tumour. J Clin Neurosci. 2007;14:1158–1162. doi: 10.1016/j.jocn.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 47.Almeida J.P., Sanchez M.M., Karekezi C., et al. Pituitary apoplexy: results of surgical and conservative management clinical series and review of the literature. World Neurosurg. 2019;130:e988–e999. doi: 10.1016/j.wneu.2019.07.055. [DOI] [PubMed] [Google Scholar]
- 48.Tang M.Y., Chen T.W., Zhang X.M., Huang X.H. GRE T2-weighted MRI: principles and clinical applications. Biomed Res Int. 2014;2014:e312142. doi: 10.1155/2014/312142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fleseriu M., Buchfelder M., Cetas J.S., et al. Pituitary society guidance: pituitary disease management and patient care recommendations during the COVID-19 pandemic—an international perspective. Pituitary. 2020;23:327–337. doi: 10.1007/s11102-020-01059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agarwal A., Fernando S.M., Honarmand K., et al. Risk of dispersion or aerosol generation and infection transmission with nasopharyngeal and oropharyngeal swabs for detection of COVID-19: a systematic review. BMJ Open. 2021;11:e040616. doi: 10.1136/bmjopen-2020-040616. [DOI] [PMC free article] [PubMed] [Google Scholar]