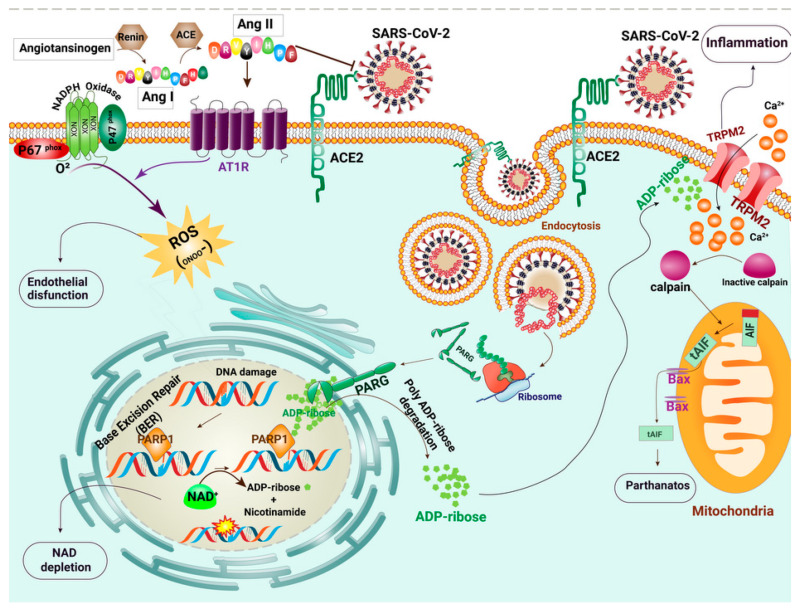
Figure 1.
Schematic of the molecular events that occur in SARS-CoV-2 infected cells. SARS-CoV-2 forms a complex with angiotensin-converting enzyme 2 (ACE2) and enters cells via cell membrane-localized receptors. Receptor occupation by SARS-CoV-2 triggers ACE2 down-regulation and renin-angiotensin system (RAS) over-activation. Renin (REN) mediates the transformation of angiotensinogen (AGT) to angiotensin I (Ang-I). The angiotensin-converting enzyme (ACE) metabolizes Ang I to angiotensin II (Ang II). Because of the reduction in available ACE2, the angiotensin type I receptor (AT1R) is activated by the elevated Ang II. Subsequently, AT1R triggers a signaling cascade via activation of NADPH oxidase and induces intense oxidative stress. The produced ROS causes the breakage of DNA strands. Base excision repair (BER) plays a crucial role in repairing oxidative DNA damage via Poly(ADP-ribose) polymerase (PARP) activity. PARP undergoes auto-modification by transferring ADP-ribose on itself. Poly(ADP-ribose) glycohydrolase (PARG) is a protein encoded by Coronaviridae, which separates ADP-ribose units from PAR. Detrimental PARP and PARG activities lead to the accumulation of ADP ribose in the cytosol. Transient receptor potential channel melastatin 2 (TRPM2) channels are over-activated by direct binding of ADP-ribose and release a large amount of calcium ions (Ca2+) to the cell. The intracellular Ca2+ overload probably results in parthanatos, a form of programmed cell death that is distinct from other cell death processes such as necrosis and apoptosis.