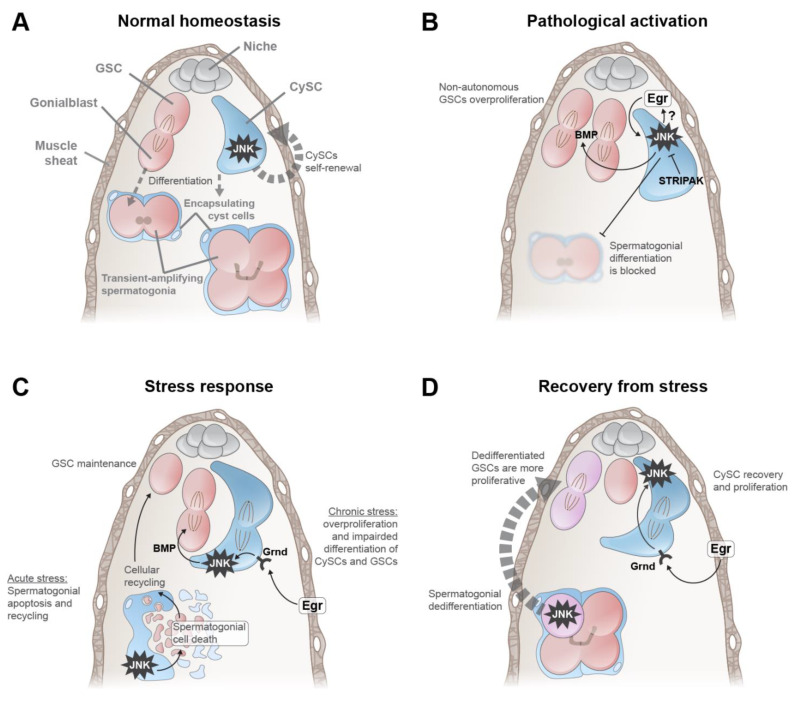
Figure 2.
Roles of the JNK pathway in the Drosophila testis. (A) Normal homeostasis. The testis is surrounded by a sheath of smooth muscle cells (brown). Niche cells (gray) are anchored to the muscle sheath and secrete self-renewal factors for GSCs (red) and CySCs (blue). A GSC divides asymmetrically to produce a GSC daughter and a differentiating daughter (termed the Gonialblast (Gb)), which undergoes transit-amplifying divisions, producing spermatogonia. A CySC divides to produce cyst cells that become quiescent and encapsulate spermatogonia and remain attached to germ cells throughout their differentiation into spermatids. During homeostasis, JNK is intrinsically activated in CySCs and promotes their self-renewal. (B) Pathological activation. Impairment of the STRIPAK complex or the endocytic pathway strongly induces JNK in somatic cells, which produce abnormally high levels of Dpp/BMP. Sustained secretion of Dpp/BMP into the testis lumen causes hyper-proliferation of GSCs and early spermatogonia, while concomitantly blocking spermatogonial differentiation. These effects are genetically dependent on Egr/TNFα, which is likely produced by CySCs and then acts in an autocrine manner. (C) Stress response. During acute stress (left), somatic JNK activity causes the non-autonomous death of spermatogonial cells, and these somatic cells endocytose and recycle the debris of the dying germ cells. This recycling is necessary for maintaining the GSC pool during stress, presumably because recycled cellular components are somehow provided to GSCs. Under chronic stress (right), the muscle sheath secretes Egr/TNFα, which is transduced by CySCs through Grnd/TNFR. This JNK activity causes hyper-proliferation of CySCs. JNK-activated CySCs secrete Dpp/BMP, which strongly induces GSC proliferation. (D) Recovery from stress. Upon termination of starvation (left), JNK autonomous activation in one or more cells in a spermatogonium (pink cell with starred JNK) induces dedifferentiation into GSCs. This dedifferentiated GSC (pink dividing cells at niche) is more proliferative than the wild-type (i.e., non-dedifferentiated) sibling GSC (red cell at niche). Additionally, upon termination of starvation (right), Egr/TNFα is secreted by the muscle sheath cells and is transduced by CySCs through Grnd/TNFR. JNK-activated CySCs increase their proliferation, which facilitates the quick recovery of the CySC pool.